International Journal of Nanomaterials, Nanotechnology and Nanomedicine
Role of Nanomaterials in Pharmaceutical Preparation: A Review
Mahendra Kumar Sahu* and Sandip Prasad Tiwari
Cite this as
Sahu MK, Tiwari SP. Role of Nanomaterials in Pharmaceutical Preparation: A Review. Int J Nanomater Nanotechnol Nanomed. 2024;10(2):056-067. Available from: 10.17352/2455-3492.000063Copyright License
© 2024 Sahu MK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Aim & objective: This study aims to explore the multifaceted applications of nanomaterials and assess their potential advantages and disadvantages. Understanding the different physicochemical properties of nanomaterials & excipients.
Method: This study reviews current literature and research findings to compile a comprehensive overview of nanomaterials. Key aspects covered include synthesis methods, characterization techniques, and notable applications in different industries. The review also discusses the advantages and disadvantages associated with nanomaterials, highlighting their potential benefits and challenges.
Advantages & disadvantages: Nanomaterials offer numerous advantages such as enhanced mechanical, electrical, and optical properties, which make them suitable for developing advanced technologies. However, challenges include concerns over their potential environmental and health impacts, as well as the scalability of production processes from laboratory to industrial scales. Additionally, the high cost of synthesis and regulatory uncertainties pose hurdles to widespread adoption.
Conclusion: In conclusion, nanomaterials present a paradigm shift in materials science and engineering with vast potential to address pressing global challenges. Their unique properties enable innovative solutions across various sectors, promising significant advancements in healthcare, electronics, energy, and environmental sustainability. However, realizing these benefits requires continued research to mitigate risks and optimize their applications. Establishing robust regulatory frameworks and fostering interdisciplinary collaborations are essential to harnessing the full potential of nanomaterials while ensuring their safe and sustainable integration into society.
Introduction
Nanomaterials are materials with at least one dimension in the nanometer scale, typically ranging from 1 to 100 nanometers. These materials exhibit unique properties due to their small size, such as increased surface area, quantum effects, and enhanced mechanical, electrical, and optical properties [1,2]. Nanomaterials can be classified into various categories based on their composition, structure, and properties. One of the most common types of nanomaterials is nanoparticles, which are particles with dimensions in the nanometer scale. Nanoparticles can be made from a variety of materials, including metals, metal oxides, carbon-based materials, and polymers. These materials have applications in fields such as catalysis, sensing, drug delivery, and imaging. Another important class of nanomaterials is nanotubes, which are cylindrical structures with nanoscale dimensions. Carbon nanotubes, in particular, have attracted significant attention due to their exceptional mechanical, electrical, and thermal properties. These materials are used in applications ranging from electronics and energy storage to aerospace and biomedical devices. Nanoporous materials are another category of nanomaterials that have a high surface area and pore volume. These materials are used in gas storage, separation, and catalysis applications. Nanocomposites are materials composed of two or more nanoscale components and are also important in various industries, including automotive, aerospace, and construction. The properties of nanomaterials are influenced by their size, shape, composition, and structure. For example, quantum effects become more pronounced at the nanoscale, leading to unique electronic and optical properties. Additionally, the high surface area-to-volume ratio of nanomaterials enhances their reactivity and adsorption capacity. Nanomaterials have a wide range of applications in various fields, including electronics, medicine, energy, and environmental remediation [3,4]. In electronics, nanomaterials are used to develop high-performance transistors, sensors, and displays. In medicine, nanomaterials are employed for drug delivery, imaging, and tissue engineering. In energy, nanomaterials play a crucial role in solar cells, batteries, and fuel cells. In environmental remediation, nanomaterials are used for water purification, air filtration, and waste treatment. Despite their numerous advantages, nanomaterials also pose challenges in terms of health and environmental risks. The small size and high reactivity of nanomaterials can lead to unintended consequences, such as toxicity and environmental pollution [4]. Therefore, it is essential to conduct thorough risk assessments and implement appropriate safety measures when working with nanomaterials. In conclusion, nanomaterials are a diverse and versatile class of materials with unique properties and a wide range of applications. As research in this field continues to advance, nanomaterials are expected to play an increasingly important role in shaping the future of technology and innovation. Nanomaterials offer several advantages over conventional materials due to their unique properties at the nanoscale. One key benefit is their increased surface area-to-volume ratio, which enhances reactivity and allows for improved performance in various applications. Additionally, nanomaterials exhibit size-dependent properties, such as enhanced mechanical strength, electrical conductivity, and optical properties, making them highly versatile for different uses. Their small size also enables precise control over material properties, leading to tailored functionalities for specific applications [5,6]. In the future, nanomaterials are expected to revolutionize industries such as healthcare, electronics, and environmental sustainability. They hold the potential to enable advancements in targeted drug delivery systems, high-performance electronics, and efficient energy storage devices. Overall, the continued development and utilization of nanomaterials are poised to drive innovation and create new opportunities across a wide range of fields.
Advantages
Nanomaterials offer several advantages over conventional materials due to their unique properties at the nanoscale. Some of the key benefits of nanomaterials compared to traditional materials include:
Enhanced mechanical properties: Nanomaterials exhibit superior mechanical strength, hardness, and flexibility compared to bulk materials. This makes them ideal for applications requiring high durability and resistance to wear and tear.
Increased surface area: Nanomaterials have a high surface area-to-volume ratio, allowing for improved reactivity and adsorption capabilities. This property is beneficial for catalysis, sensing, and filtration applications [2,7].
Enhanced electrical and optical properties: Nanomaterials possess unique electrical and optical properties, such as quantum confinement effects and plasmonic behavior. These properties enable the development of advanced electronic devices, sensors, and photonic technologies.
Tailored chemical and physical properties: Nanomaterials can be engineered to exhibit specific chemical, physical, and biological properties by controlling their size, shape, and composition. This tunability allows for custom-designed materials with desired functionalities [7].
Biocompatibility and bioactivity: Some nanomaterials are biocompatible and exhibit bioactive properties, making them suitable for biomedical applications such as drug delivery, imaging, and tissue engineering.
Environmental benefits: Nanomaterials can be used for environmental remediation, pollution control, and sustainable energy applications. They offer efficient catalytic properties, adsorption capabilities, and photocatalytic activity for environmental cleanup and energy conversion processes [8].
Future benefits
In terms of future benefits, nanomaterials hold great potential for various industries and technologies. Some of the anticipated future benefits of nanomaterials include:
Advanced healthcare solutions: Nanomaterials are expected to revolutionize healthcare by enabling targeted drug delivery, personalized medicine, and non-invasive diagnostics. They can enhance the efficacy and safety of medical treatments while reducing side effects [9].
Energy storage and conversion: Nanomaterials are poised to improve energy storage devices like batteries and supercapacitors by enhancing their performance, durability, and efficiency. They also hold promise for renewable energy technologies such as solar cells and fuel cells.
Smart materials and devices: Nanomaterials can be integrated into smart materials and devices with responsive, adaptive, and self-healing properties. These materials have applications in robotics, electronics, aerospace, and other high-tech industries [10,11].
Environmental sustainability: Nanomaterials offer sustainable solutions for environmental challenges, including water purification, air filtration, waste treatment, and renewable energy generation. They can contribute to a cleaner and greener future. Overall, nanomaterials have the potential to drive innovation, improve quality of life, and address global challenges in diverse fields. Continued research and development in nanotechnology will unlock new opportunities for harnessing the benefits of nanomaterials in the years to come [12].
Types of nanomaterials preparation
Carbon nanotubes: Carbon nanotubes are cylindrical structures composed of carbon atoms arranged in a hexagonal lattice. They exhibit unique properties such as high strength, flexibility, and electrical conductivity, making them valuable in various fields including electronics, materials science, and nanotechnology. One important detail about carbon nanotubes is their exceptional mechanical strength. They are one of the strongest materials known, with a tensile strength exceeding that of steel [13]. This property makes them ideal for reinforcing composite materials and creating lightweight yet durable structures. Another key aspect of carbon nanotubes is their electrical conductivity. They can conduct electricity better than copper, making them suitable for applications in electronics and nanoelectronics. Carbon nanotubes have been used in the development of high-performance transistors, sensors, and conductive films. Furthermore, carbon nanotubes have a high aspect ratio, meaning they have a large surface area relative to their volume. This property makes them effective in applications such as energy storage devices, catalysis, and drug delivery systems. In summary, carbon nanotubes possess remarkable mechanical strength, electrical conductivity, and high aspect ratio, making them versatile materials with a wide range of potential applications in various industries [6].
Graphene: Graphene is a two-dimensional material composed of a single layer of carbon atoms arranged in a hexagonal lattice. It is known for its exceptional properties, including high electrical conductivity, mechanical strength, and thermal conductivity. One important detail about graphene is its electrical conductivity. Graphene is an excellent conductor of electricity, surpassing even copper and other traditional conductive materials. This property makes graphene valuable in the development of high-speed electronics, sensors, and transparent conductive films. Another key aspect of graphene is its mechanical strength. Despite being only one atom thick, graphene is incredibly strong and flexible. It has a tensile strength greater than that of steel, making it suitable for reinforcing composite materials and creating lightweight yet durable structures. Graphene also exhibits outstanding thermal conductivity, allowing it to efficiently dissipate heat [14]. This property is essential for applications in thermal management, such as in electronic devices and energy storage systems. Furthermore, graphene's large surface area and unique electronic properties make it promising for applications in energy storage, catalysis, and biomedical devices. In summary, graphene's exceptional electrical conductivity, mechanical strength, thermal conductivity, and unique electronic properties make it a highly versatile material with a wide range of potential applications in various industries [15,16].
Quantum dots: Quantum dots are nanoscale semiconductor particles that exhibit unique optical and electronic properties due to quantum confinement effects. These tiny structures are typically composed of materials such as cadmium selenide, lead sulfide, or indium arsenide. One important detail about quantum dots is their size-dependent optical properties. The size of a quantum dot directly influences its bandgap, which in turn determines the color of light it emits. This tunability makes quantum dots valuable for applications in displays, lighting, and biological imaging. Another key aspect of quantum dots is their high photoluminescence efficiency. When excited by light or electricity, quantum dots emit bright, colorful light with narrow emission spectra. This property is advantageous for producing vibrant and energy-efficient displays, as well as for use in sensors and medical imaging. Furthermore, quantum dots have unique electronic properties that can be tailored for specific applications [17]. They can be engineered to exhibit properties such as high charge carrier mobility, making them promising for use in transistors, solar cells, and quantum computing. Additionally, quantum dots have shown potential in the field of quantum cryptography and quantum information processing due to their ability to generate entangled photon pairs and exhibit quantum coherence. In summary, quantum dots' size-dependent optical properties, high photoluminescence efficiency, tunable electronic properties, and potential for quantum technologies make them a versatile and promising material with a wide range of applications in areas such as displays, lighting, sensors, and quantum computing [7,18].
Metal nanoparticles: Metal nanoparticles are nanoscale particles composed of metal atoms, typically ranging in size from 1 to 100 nanometers. These nanoparticles exhibit unique physical and chemical properties that differ from their bulk counterparts, making them valuable in various applications across different industries. One important detail about metal nanoparticles is their high surface area-to-volume ratio. Due to their small size, metal nanoparticles have a significantly larger surface area compared to their volume [19]. This property enhances their reactivity and makes them effective catalysts in chemical reactions, such as in the production of fine chemicals and pharmaceuticals. Another key aspect of metal nanoparticles is their optical properties. These nanoparticles can exhibit localized surface plasmon resonance, a phenomenon where the metal's free electrons collectively oscillate in response to incident light. This property gives metal nanoparticles unique optical characteristics, making them useful in applications such as sensing, imaging, and photothermal therapy [20]. Metal nanoparticles also possess excellent electrical and thermal conductivity, making them suitable for use in electronics, sensors, and energy storage devices. Their small size and tunable properties allow for the design of advanced materials with enhanced performance. Furthermore, metal nanoparticles have antimicrobial properties and are used in various biomedical applications, including drug delivery, imaging, and cancer therapy. In summary, metal nanoparticles offer a wide range of unique properties and applications due to their small size and distinctive characteristics. Their high surface area-to-volume ratio, optical properties, conductivity, and antimicrobial effects make them valuable materials in fields such as catalysis, electronics, biomedicine, and environmental remediation [21,22].
Nanowires: Nanowires are one-dimensional structures with diameters on the nanometer scale and lengths that can range from micrometers to millimeters. They are typically made of various materials such as metals, semiconductors, or insulators and exhibit unique properties that make them valuable in nanotechnology and electronics. One important detail about nanowires is their high aspect ratio. Due to their small diameter and elongated shape, nanowires have a large surface area relative to their volume. This property makes them ideal for applications such as sensors, catalysts, and energy storage devices where surface interactions are crucial. Another key aspect of nanowires is their tunable electrical and optical properties [23]. By controlling the composition, size, and structure of nanowires, researchers can tailor their electrical conductivity, bandgap, and optical properties for specific applications. This versatility makes nanowires promising for use in nanoelectronics, photovoltaics, and optoelectronics. Nanowires also exhibit mechanical flexibility and strength, allowing them to be integrated into flexible electronics and nanomechanical devices. Their small size and high surface-to-volume ratio enable efficient energy conversion and storage in nanoscale devices. Furthermore, nanowires can serve as building blocks for nanoscale devices and systems, enabling the development of novel technologies such as nanoscale sensors, transistors, and nanolasers. In summary, nanowires' high aspect ratio, tunable properties, mechanical flexibility, and versatility as building blocks make them valuable materials for a wide range of applications in nanotechnology, electronics, and materials science [24].
Nanoparticles: Nanoparticles are particles with dimensions ranging from 1 to 100 nanometers in at least one dimension. They exhibit unique properties due to their small size, large surface area-to-volume ratio, and quantum effects. One important detail about nanoparticles is their size-dependent properties. As the size of nanoparticles decreases, their physical and chemical properties can change significantly [25,26]. This size-dependent behavior makes nanoparticles valuable in various applications, such as catalysis, drug delivery, and imaging. Another key aspect of nanoparticles is their large surface area-to-volume ratio. This property allows nanoparticles to interact more effectively with surrounding molecules, making them efficient catalysts, sensors, and drug carriers. The high surface area also enhances the reactivity and adsorption capacity of nanoparticles. Nanoparticles also exhibit quantum effects at the nanoscale. These effects can lead to unique optical, electronic, and magnetic properties that differ from those of bulk materials. Quantum dots, for example, are semiconductor nanoparticles that emit light of specific wavelengths based on their size, making them useful in displays, imaging, and biological labeling. Furthermore, nanoparticles can be engineered with specific shapes, compositions, and surface modifications to tailor their properties for specific applications. This versatility allows nanoparticles to be utilized in a wide range of fields, including medicine, electronics, environmental remediation, and energy storage. In summary, nanoparticles possess size-dependent properties, a large surface area-to-volume ratio, quantum effects, and tunable characteristics, making them versatile materials with diverse applications in science, technology, and industry [27].
Nanomaterials in the pharmaceutical industry
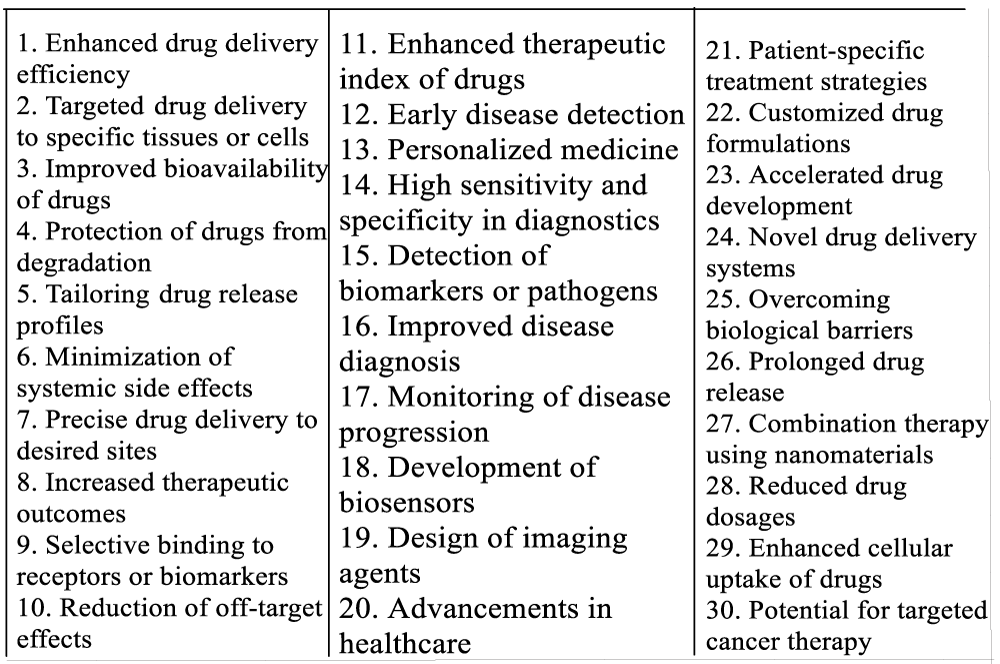
Nanomaterials play a crucial role in pharmaceutical preparation due to their unique properties and potential applications in drug delivery, diagnostics, and therapeutics [2,7]. These materials, typically ranging in size from 1 to 100 nanometers, offer several advantages in the field of medicine. One key role of nanomaterials in pharmaceutical preparation is their ability to enhance drug delivery efficiency. Nanoparticles can encapsulate drugs, protecting them from degradation and improving their bioavailability. By controlling the size, shape, and surface properties of nanoparticles, researchers can tailor drug release profiles and target specific tissues or cells, leading to improved therapeutic outcomes. Moreover, nanomaterials enable targeted drug delivery, reducing systemic side effects and improving treatment efficacy [13,28]. Functionalized nanoparticles can be designed to selectively bind to specific receptors or biomarkers on diseased cells, allowing for precise drug delivery to the desired site. This targeted approach minimizes off-target effects and enhances the therapeutic index of drugs. In addition to drug delivery, nanomaterials have revolutionized diagnostics in pharmaceutical preparation. Nanoparticles can be engineered to detect biomarkers or pathogens with high sensitivity and specificity, enabling early disease detection and personalized medicine. Nanotechnology-based diagnostic tools, such as biosensors and imaging agents, have significantly improved disease diagnosis and monitoring [2,13]. Overall, the role of nanomaterials in pharmaceutical preparation is multifaceted, encompassing drug delivery, targeted therapy, and diagnostics given in Figure 1.
Common excipients used in nanoparticle formulation
Nanoparticle formulation includes stabilizers like surfactants (e.g., Poloxamer, Tween), polymers (e.g., polyethylene glycol, PLGA), and cryoprotectants (e.g., sugars like sucrose). These components help stabilize nanoparticles, control particle size, enhance drug loading, and improve biocompatibility and stability during formulation and storage [28,29].
Polymers: Large molecules composed of repeating structural units known as monomers. There are various types of polymers, including synthetic polymers like polyethylene, polypropylene, and polystyrene, as well as natural polymers like proteins, cellulose, and DNA. Polymers play a crucial role in numerous industries and applications due to their diverse functions and properties [28]. One of the key advantages of polymers is their versatility and tunable properties. By varying the monomer composition, molecular weight, and structure, polymers can be tailored to meet specific requirements for different applications. For example, polymers can be engineered to exhibit desired mechanical strength, flexibility, thermal stability, and chemical resistance, making them suitable for a wide range of uses in industries such as packaging, automotive, construction, and healthcare [11,28,30].
Additionally, polymers offer advantages such as lightweight, cost-effectiveness, and ease of processing. Compared to traditional materials like metals and ceramics, polymers are often lighter in weight, which is advantageous for applications where weight reduction is critical, such as in the aerospace and automotive industries. Moreover, polymers can be produced in large quantities at relatively low cost, making them economically viable for mass production. The processability of polymers also allows for efficient manufacturing through techniques like injection molding, extrusion, and 3D printing. However, along with their numerous advantages, polymers also have certain disadvantages that need to be considered. One of the main drawbacks of some polymers is their susceptibility to degradation under environmental conditions such as exposure to UV radiation, heat, and chemicals [2,7]. This can lead to issues like discoloration, embrittlement, and loss of mechanical properties over time. Furthermore, some polymers may pose environmental concerns due to their non-biodegradable nature, contributing to plastic pollution and waste accumulation. In conclusion, polymers encompass a wide range of materials with diverse types, functions, advantages, and disadvantages [28,31]. Their versatility, tunable properties, and cost-effectiveness make them indispensable in various industries and applications. However, challenges such as environmental impact and degradation issues highlight the importance of sustainable polymer design and recycling practices to mitigate potential drawbacks. By understanding the characteristics and behavior of different polymer types, researchers and industries can continue to innovate and optimize the use of polymers for a sustainable and efficient future [22,28,32] given in Table 1.
Surfactants: Compounds that play a crucial role in various industries and everyday products due to their unique properties. There are several types of surfactants, including anionic, cationic, nonionic, and amphoteric surfactants, each with distinct characteristics and functions. Anionic surfactants, such as sodium lauryl sulfate, are commonly used in cleaning products for their excellent foaming and emulsifying properties. Cationic surfactants, like cetyltrimethylammonium bromide, are often found in hair conditioners and fabric softeners due to their ability to provide a positive charge and improve product performance. Nonionic surfactants, such as polysorbate 80, are known for their compatibility with various substances and are frequently used in pharmaceuticals and personal care products [43]. Amphoteric surfactants, like cocamidopropyl betaine, exhibit both positive and negative charges, making them versatile and suitable for a wide range of applications. Surfactants serve several essential functions in products and processes. They act as emulsifiers, helping to mix oil and water-based ingredients in formulations like lotions and creams. Surfactants also function as detergents, reducing the surface tension of liquids to facilitate the removal of dirt and grease. In addition, surfactants can stabilize emulsions, preventing the separation of immiscible components in products like salad dressings and cosmetics. Furthermore, surfactants play a crucial role in wetting agents, allowing liquids to spread evenly on surfaces, and enhancing cleaning efficiency. Overall, surfactants contribute to the effectiveness, stability, and performance of a wide range of products across industries [7,44].
Despite their numerous advantages, surfactants also have some disadvantages that need to be considered. One common concern is their potential environmental impact, as certain surfactants can be toxic to aquatic life and may persist in the environment. Additionally, some surfactants can cause skin irritation or allergic reactions in sensitive individuals, highlighting the importance of proper safety precautions and labeling in product formulations. Surfactants may also contribute to foam pollution in water bodies, affecting aquatic ecosystems and water quality [45]. Furthermore, the production and disposal of surfactants can have energy and resource implications, necessitating sustainable practices and alternatives to minimize environmental harm. In conclusion, surfactants play a vital role in a wide range of applications, offering unique properties and functions that enhance the performance of products and processes. Understanding the different types of surfactants, their functions, advantages, and disadvantages is essential for formulators, manufacturers, and consumers to make informed decisions regarding product development, usage, and environmental impact. By balancing the benefits and drawbacks of surfactants and adopting responsible practices, stakeholders can harness the potential of these versatile compounds while minimizing their potential negative effects on health and the environment given in Table 2 [22,46].
Lipids: A diverse group of organic compounds that play essential roles in the human body. There are several types of lipids, including triglycerides, phospholipids, sterols, and sphingolipids, each with distinct structures and functions. Triglycerides, the most common type of lipid, serve as a major energy source and storage form of fat in the body. They provide insulation and protection for organs, as well as serving as precursors for various hormones. Phospholipids are crucial components of cell membranes, forming a lipid bilayer that regulates the passage of molecules in and out of cells. Sterols, such as cholesterol, are important for cell membrane structure, hormone synthesis, and bile acid production. Sphingolipids are involved in cell signaling and cell recognition processes [22]. The advantages of lipids in the body are numerous. They provide a concentrated source of energy, with each gram of fat containing more than twice the calories of carbohydrates or proteins. Lipids also aid in the absorption of fat-soluble vitamins (A, D, E, and K) and help maintain healthy skin and hair. Additionally, lipids play a crucial role in cell signaling, serving as messengers that regulate various physiological processes in the body. Certain types of lipids, such as omega-3 fatty acids, have anti-inflammatory properties and are beneficial for heart health [7,54].
Despite their important functions, lipids can also have disadvantages when consumed in excess. High intake of saturated fats and trans fats, commonly found in processed foods, can lead to an increased risk of cardiovascular diseases, obesity, and other health issues. Excessive consumption of cholesterol-rich foods can contribute to the development of atherosclerosis and heart disease. It is essential to maintain a balanced diet that includes healthy fats, such as monounsaturated and polyunsaturated fats while limiting the intake of unhealthy fats to promote overall health and well-being. In conclusion, lipids are vital components of a healthy diet and play diverse roles in the human body [53,55]. Understanding the different types of lipids, their functions, advantages, and potential disadvantages can help individuals make informed dietary choices to support optimal health and well-being. By incorporating a variety of healthy fats into their diet and avoiding excessive consumption of unhealthy fats, individuals can harness the benefits of lipids while minimizing potential risks to their health as shown in Table 3.
Chelating agents: Chemical compounds that have the ability to form coordination complexes with metal ions by donating electron pairs to the metal ion. These compounds play a crucial role in various industrial, environmental, and biological processes due to their unique properties. There are several types of chelating agents, including ethylenediaminetetraacetic acid (EDTA), citric acid, diethylenetriaminepentaacetic acid (DTPA), and hydroxycarboxylic acids like gluconic acid. Each type of chelating agent has specific characteristics that make them suitable for different applications. The primary function of chelating agents is to sequester metal ions and form stable complexes, thereby preventing the metal ions from participating in undesired chemical reactions [17,28]. Chelation can be used in various industries such as water treatment, food preservation, and pharmaceuticals to control the presence of metal ions and improve product stability. Chelating agents also find applications in medicine, where they are used to treat heavy metal poisoning by forming complexes with toxic metal ions and facilitating their excretion from the body. One of the key advantages of using chelating agents is their ability to enhance the stability and solubility of metal ions in solution. By forming stable complexes with metal ions, chelating agents can prevent the precipitation of insoluble metal salts and improve the efficiency of various chemical processes. Additionally, chelating agents can act as corrosion inhibitors by forming protective layers on metal surfaces, thereby extending the lifespan of equipment and infrastructure [60].
However, chelating agents also have certain disadvantages that need to be considered. One potential drawback is the environmental impact of chelating agents, as some compounds may persist in the environment and pose risks to ecosystems. Additionally, chelating agents can sometimes exhibit selectivity towards specific metal ions, which may limit their effectiveness in certain applications. Furthermore, the use of chelating agents in food and pharmaceutical products may raise concerns about potential health risks associated with prolonged exposure to these compounds. In conclusion, chelating agents play a vital role in various industries and applications by sequestering metal ions and forming stable complexes. Different types of chelating agents offer unique advantages and disadvantages, making them suitable for specific purposes. Understanding the properties and functions of chelating agents is essential for optimizing their use in different processes while minimizing potential risks. Further research and development in chelation chemistry can lead to the discovery of novel chelating agents with improved efficiency and reduced environmental impact given in Table 4 [61].
Conclusion
In conclusion, nanomaterials stand at the forefront of scientific innovation, promising transformative advancements across numerous fields from healthcare to electronics, energy, and environmental sustainability. Currently, these materials have already demonstrated their potential through applications such as targeted drug delivery systems in medicine, enhanced electronic properties in nanoelectronics, and improved efficiency in energy storage and conversion technologies. Looking forward, the future prospects for nanomaterials appear exceptionally bright. Anticipated developments include personalized medicine enabled by nanoscale biosensors, quantum leaps in computing through quantum dots, and smart materials that respond dynamically to their environment. These advancements not only promise to revolutionize industries but also hold the key to addressing global challenges such as clean energy production, environmental remediation, and sustainable manufacturing. However, achieving these future potentials requires addressing critical challenges such as ensuring the safety of nanomaterials, scaling up production methods, and addressing ethical and regulatory concerns. By navigating these challenges with rigorous research, collaboration, and responsible governance, nanotechnology stands poised to redefine possibilities and drive innovation toward a more sustainable and technologically advanced future.
- Wibowo YG, Ramadan BS, Taher T, Khairurrijal K. Advancements of Nanotechnology and Nanomaterials in Environmental and Human Protection for Combatting the COVID-19 During and Post-pandemic Era: A Comprehensive Scientific Review. Biomed Mater Devices. 2023;1-24. Available from: https://doi.org/10.1007/s44174-023-00086-9
- Sahu MK, Nayak AK, Hailemeskel B, Eyupoglu OE. Exploring Recent Updates on Molecular Docking: Types, Method, Application, Limitation & Future Prospects. Int J Pharm Res Allied Sci. 2024;13(2-2024):24-40. Available from: https://doi.org/10.51847/Une9jqjUCl
- Sahu MK. A review on glaucoma: causes, symptoms, pathogenesis & treatment. J Clin Res Ophthalmol. 2024 Mar 7;11(1):001-004. Available from: https://dx.doi.org/10.17352/2455-1414.000102
- Sahu MK, Tiwari SP. Epidemiology, Pathogenesis and Treatment of Diabetes: A Comprehensive Review. World J Diabetes Res Pract. 2024;1(1):01-09. Available from: https://mkscienceset.com/articles_file/573-_article1708434044.pdf
- Sahu B, Sahu M, Sahu M, Yadav M, Sahu R, Sahu C. An Updated Review on Nelumbo Nucifera Gaertn: Chemical Composition, Nutritional Value and Pharmacological Activities. Chem Biodivers. 2024;21:e202301493. Available from: https://doi.org/10.1002/cbdv.202301493
- Chausali N, Saxena J, Prasad R. Nanotechnology as a sustainable approach for combating the environmental effects of climate change. J Agric Food Res. 2023;12:100541. Available from: https://doi.org/10.1016/j.jafr.2023.100541
- Sahu MK, Yadav R, Tiwari SP. Recent advances in nanotechnology. Int J Nanomater Nanotechnol Nanomed. 2023;9(1):015-23. Available from: https://doi.org/10.17352/2455-3492.000053
- Kumar R, Kumar M, Luthra G. Fundamental approaches and applications of nanotechnology: A mini review. Mater Today Proc. 2023. Available from: https://doi.org/10.1016/j.matpr.2022.12.172
- Mekuye B, Abera B. Nanomaterials: An overview of synthesis, classification, characterization, and applications. Nano Select. 2023;4(8):486-501. Available from: https://doi.org/10.1002/nano.202300038
- Sahu MK, Dubey N, Pandey R, Shukla SS, Gidwani B. Formulation, evaluation, and validation of microspheres of cyclophosphamide for topical delivery. Pharmacophore. 2023;14(1-2023):1-8. Available from: https://doi.org/10.51847/e4GvuoN96z
- Nagare SC, Sonwane DP, Pawar VV. A review on the use of nanotechnology and nanomaterials in civil engineering. International Journal of Innovative Research in Science and Engineering. 2017;3(03):272-8. Available from: https://ijirse.com/wp-content/upload/2017/03/N1024ijirse.pdf
- Malik S, Muhammad K, Waheed Y. Emerging applications of nanotechnology in healthcare and medicine. Molecules. 2023;28(18):6624. Available from: https://doi.org/10.3390/molecules28186624
- Sahu MK, Tiwari SP. A Systemic Review on Recent Outbreaks of Dengue and Newer US FDA Approved Drug. J Infec Dise and Vir Res. 2023;2(2):6. Available from: https://mkscienceset.com/articles_file/575-_article1687167234.pdf
- Alrushaid N, Khan FA, Al-Suhaimi EA, Elaissari A. Nanotechnology in cancer diagnosis and treatment. Pharmaceutics. 2023;15(3):1025. Available from: https://doi.org/10.3390/pharmaceutics15031025
- Singh R, Dutt S, Sharma P, Sundramoorthy AK, Dubey A, Singh A, et al. Future of nanotechnology in food industry: Challenges in processing, packaging, and food safety. Global Challenges. 2023;7(4):2200209. Available from: https://doi.org/10.1002/gch2.202200209
- Gajanan K, Tijare SN. Applications of nanomaterials. Materials Today: Proceedings. 2018;5(1):1093-1096. Available from: https://doi.org/10.1016/j.matpr.2017.11.187
- Debbarma K, Debnath B, Sarkar PP. A comprehensive review on the usage of nanomaterials in asphalt mixes. Construction and Building Materials. 2022;361:129634. Available from: https://doi.org/10.1016/j.conbuildmat.2022.129634
- An C, Sun C, Li N, Huang B, Jiang J, Shen Y, et al. Nanomaterials and nanotechnology for the delivery of agrochemicals: strategies towards sustainable agriculture. Journal of Nanobiotechnology. 2022;20(1):11. Available from: https://doi.org/10.1186/s12951-021-01214-7
- Gupta V, Mohapatra S, Mishra H, Farooq U, Kumar K, Ansari MJ, et al. Nanotechnology in cosmetics and cosmeceuticals—a review of latest advancements. Gels. 2022;8(3):173. Available from: https://doi.org/10.3390/gels8030173
- Singh AR, Desu PK, Nakkala RK, Kondi V, Devi S, Alam MS, Hamid H, et al. Nanotechnology-based approaches applied to nutraceuticals. Drug Deliv Transl Res. 2022;12(3):485-499. Available from: https://doi.org/10.1007/s13346-021-00960-3
- Singh KK, Singh A, Rai S. A study on nanomaterials for water purification. Mater Today Proc. 2022;51:1157-63. Available from: https://doi.org/10.1016/j.matpr.2021.07.116
- Fazal-ur-Rehman M. Novel applications of nanomaterials and nanotechnology in medical sciences-a review. J Basic Appl Sci Res. 2018;8(4):1. Available from: https://www.researchgate.net/profile/Fazal-Ur-Rehman-13/publication/324331262_Novel_Applications_of_Nanomaterials_and_Nanotechnology_in_Medical_Sciences-A_Review/links/5ad2c643aca272fdaf799b75/Novel-Applications-of-Nanomaterials-and-Nanotechnology-in-Medical-Sciences-A-Review.pdf
- Mosleh-Shirazi S, Abbasi M, Moaddeli MR, Vaez A, Shafiee M, Kasaee SR, et al. Nanotechnology advances in the detection and treatment of cancer: an overview. Nanotheranostics. 2022;6(4):400-423. Available from: https://doi.org/10.7150/ntno.74613
- Chausali N, Saxena J, Prasad R. Recent trends in nanotechnology applications of bio-based packaging. J Agric Food Res. 2022;7:100257. Available from: https://doi.org/10.1016/j.jafr.2021.100257
- Beni AA, Jabbari H. Nanomaterials for environmental applications. Results Eng. 2022;15:100467. Available from: https://doi.org/10.1016/j.rineng.2022.100467
- Xue Z, Zhang Y, Yu W, Zhang J, Wang J, Wan F, et al. Recent advances in aflatoxin B1 detection based on nanotechnology and nanomaterials-A review. Anal Chim Acta. 2019;1069:1-27. Available from: https://doi.org/10.1016/j.aca.2019.04.032
- Dubey SK, Dey A, Singhvi G, Pandey MM, Singh V, Kesharwani P. Emerging trends of nanotechnology in advanced cosmetics. Colloids Surf B Biointerfaces. 2022;214:112440. Available from: https://doi.org/10.1016/j.colsurfb.2022.112440
- Sahu MK, Singh VK, Rao SP. Development and evaluation of antidiabetic potential of polyherbal formulation in streptozotocin induced animal model. Int J Cell Sci Mol Biol. 2018;5(2):0029-37. Available from: https://ideas.repec.org/a/adp/ijcsmb/v5y2018i1p29-37.html
- Al-Mamoori SO, Jabuk SI, Mahdi RK, Naji NM, Almaamori AM. The use of nanotechnology in medicine. Eur J Res Dev Sustain. 2022;3:115-20. Available from: https://www.researchgate.net/profile/Anmar-Mahdi-Almaamori/publication/373434826_THE_USE_OF_NANOTECHNOLOGY_IN_MEDICINE/links/64eb94720453074fbdb811b3/THE-USE-OF_NANOTECHNOLOGY_IN_MEDICINE.pdf
- Tahir MB, Sohaib M, Sagir M, Rafique M. Role of nanotechnology in photocatalysis. In: Encyclopedia of Smart Materials. 2022:578-589. Available from: https://doi.org/10.1016/B978-0-12-815732-9.00006-1
- Arora S, Murmu G, Mukherjee K, Saha S, Maity D. A comprehensive overview of nanotechnology in sustainable agriculture. J Biotechnol. 2022;355:21-41. Available from: https://doi.org/10.1016/j.jbiotec.2022.06.007
- Ojha B, Jain VK, Mehra NK, Jain K. Nanotechnology: Introduction and basic concepts. In: Dendrimers in Nanomedicine. CRC Press; 2021;1-17. Available from: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003029915-1/nanotechnology-introduction-basic-concepts-brijesh-ojha-vineet-kumar-jain-neelesh-kumar-mehra-keerti-jain
- Okey‐Onyesolu CF, Hassanisaadi M, Bilal M, Barani M, Rahdar A, Iqbal J, et al. Nanomaterials as nanofertilizers and nanopesticides: an overview. ChemistrySelect. 2021;6(33):8645-8663. Available from: https://doi.org/10.1002/slct.202102379
- Iravani S. Nanomaterials and nanotechnology for water treatment: recent advances. Inorg Nano-Metal Chem. 2021 Sep 29;51(12):1615-1645. Available from: https://doi.org/10.1080/24701556.2020.1852253
- Gottardo S, Mech A, Drbohlavová J, Małyska A, Bøwadt S, Sintes JR, et al. Towards safe and sustainable innovation in nanotechnology: State-of-play for smart nanomaterials. NanoImpact. 2021;21:100297. Available from: https://doi.org/10.1016/j.impact.2021.100297
- Kumar R, Aadil KR, Ranjan S, Kumar VB. Advances in nanotechnology and nanomaterials based strategies for neural tissue engineering. J Drug Deliv Sci Technol. 2020;57:101617. Available from: https://doi.org/10.1016/j.jddst.2020.101617
- Jandt KD, Watts DC. Nanotechnology in dentistry: Present and future perspectives on dental nanomaterials. Dent Mater. 2020;36(11):1365-1378. Available from: https://doi.org/10.1016/j.dental.2020.08.006
- Bodzek M, Konieczny K, Kwiecińska-Mydlak A. Nanotechnology in water and wastewater treatment. Graphene–the nanomaterial for next generation of semipermeable membranes. Crit Rev Environ Sci Technol. 2020 Aug 2;50(15):1515-1579. Available from: https://doi.org/10.1080/10643389.2019.1664258
- Inshakova E, Inshakova A. Nanomaterials and nanotechnology: prospects for technological re-equipment in the power engineering industry. IOP Conf Ser Mater Sci Eng. 2020;709(3):033020. Available from: https://iopscience.iop.org/article/10.1088/1757-899X/709/3/033020/meta
- Jameel AT, Yaser AZ, editors. Advances in nanotechnology and its applications. Springer; 2020 Aug 27. Available from: https://link.springer.com/chapter/10.1007/978-981-15-4742-3_1
- Saleem H, Zaidi SJ. Sustainable use of nanomaterials in textiles and their environmental impact. Materials. 2020;13(22):5134. Available from: https://doi.org/10.3390/ma13225134
- Kumar H, Kuča K, Bhatia SK, Saini K, Kaushal A, Verma R, et al. Applications of nanotechnology in sensor-based detection of foodborne pathogens. Sensors. 2020;20(7):1966. Available from: https://doi.org/10.3390/s20071966
- Khanal LR, Sundararajan JA, Qiang Y. Advanced nanomaterials for nuclear energy and nanotechnology. Energy Technology. 2020;8(3):1901070. Available from: https://doi.org/10.1002/ente.201901070
- Vázquez-Núñez E, Molina-Guerrero CE, Peña-Castro JM, Fernández-Luqueño F, de la Rosa-Álvarez MG. Use of nanotechnology for the bioremediation of contaminants: A review. Processes. 2020;8(7):826. Available from: https://doi.org/10.3390/pr8070826
- Bissessur R. Nanomaterials applications. In: Polymer Science and Nanotechnology. Elsevier. 2020;435-453.
- Malik R, Patil S. Nanotechnology: Regulatory outlook on nanomaterials and nanomedicines in United States, Europe and India. Appl Clin Res Clin Trials Reg Aff. 2020;7(3):225-36. Available from: https://doi.org/10.2174/2213476X06666191129094236
- Prasad J, Netam AK, Singh R, Sahu M, Satapathy T, Rao SP, et al. Therapeutic Approaches for the Management of Parkinson's disease. Res J Pharmacol Pharmacodyn. 2019;11(1):46-52. Available from: http://dx.doi.org/10.5958/2321-5836.2019.00009.0
- Jain S, Saraf S, Sahu MK. Recent Trends and Strategies for Targeting M-cells via Oral Vaccine against Hepatitis B: A Review. Int J Cell Sci Mol Biol. 2019;5(5):86-92. Available from: https://ideas.repec.org/a/adp/ijcsmb/v5y2019i5p86-92.html
- Zafar MS, Alnazzawi AA, Alrahabi M, Fareed MA, Najeeb S, Khurshid Z. Nanotechnology and nanomaterials in dentistry. In: Advanced Dental Biomaterials. 2019;477-505. Available from: https://doi.org/10.1016/B978-0-08-102476-8.00018-9
- Jones W, Gibb A, Goodier C, Bust P, Song M, Jin J. Nanomaterials in construction - what is being used, and where? Proc Inst Civil Eng Constr Mater. 2019;172(2):49-62. Available from: https://doi.org/10.1680/jcoma.16.00011
- Zhang Y, Chen X. Nanotechnology and nanomaterial-based no-wash electrochemical biosensors: from design to application. Nanoscale. 2019;11(41):19105-191018. Available from: https://doi.org/10.1039/C9NR05696C
- Nasrollahzadeh M, Sajadi SM, Sajjadi M, Issaabadi Z. An introduction to nanotechnology. In: Interface Science and Technology. Elsevier; 2019;1-27. Available from: https://doi.org/10.1016/B978-0-12-813586-0.00001-8
- Zaib S, Iqbal J. Nanotechnology: Applications, techniques, approaches, & the advancement in toxicology and environmental impact of engineered nanomaterials. Importance & Applications of Nanotechnology. 2019;8. Available from: https://meddocsonline.org/ebooks/ebook-nanotechnology/Importance-Application-of-Nanotechnology_GP_11_06_2019.pdf
- Lazzara G, Fakhrullin RF, editors. Nanotechnologies and nanomaterials for diagnostic, conservation and restoration of cultural heritage. Elsevier; 2018. Available from: https://books.google.co.in/books?hl=en&lr=&id=FIJyDwAAQBAJ&oi=fnd&pg=PP1&dq=54.%09Lazzara+G,+Fakhrullin+RF,+editors.+Nanotechnologies+and+nanomaterials+for+diagnostic,+conservation+and+restoration+of+cultural+heritage.+Elsevier%3B+2018+Oct+12&ots=tjTNOvWnT0&sig=21BwCDkmBAPPSR80vUMfltMgrvM&redir_esc=y#v=onepage&q=2018&f=false
- Hadef F. An introduction to nanomaterials. In: Environmental Nanotechnology: 2018;1:1-58. https://link.springer.com/chapter/10.1007/978-3-319-76090-2_1
- Brahamdutt VK, Kumar A, Hooda MS, Sangwan P. Nanotechnology: Various methods used for preparation of Nanomaterials. Asian J Pharm Pharmacol. 2018;4(4):386-93. Available from: https://www.researchgate.net/profile/Braham-Dutt/publication/331501320_Nanotechnology_Various_methods_used_for_preparation_of_Nanomaterials/links/5c7d6d94299bf1268d390a9c/Nanotechnology-Various-methods-used-for-preparation-of-Nanomaterials.pdf
- Guerra FD, Attia MF, Whitehead DC, Alexis F. Nanotechnology for environmental remediation: materials and applications. Molecules. 2018 Jul 18;23(7):1760. Available from: https://doi.org/10.3390/molecules23071760
- Thakur P, Bhardwaj KK, Gupta R. Nanotechnology and Nanomaterials: Applications and Environmental Issues. In: Green and Sustainable Advanced Materials: Applications. 2018;2:85-110. Available from: https://onlinelibrary.wiley.com/doi/book/10.1002/9781119528463#page=100
- Singh VK, Sahu MK. Antidiabetic Medicinal Plants Having Insulin Mimetic Property: A Review. Kenkyu J Pharm Pract Health Care. 2018;60:56-60.
- Corsi I, Winther-Nielsen M, Sethi R, Punta C, Della Torre C, Libralato G, et al. Ecofriendly nanotechnologies and nanomaterials for environmental applications: Key issue and consensus recommendations for sustainable and ecosafe nanoremediation. Ecotoxicol Environ Saf. 2018;154:237-44. Available from: https://doi.org/10.1016/j.ecoenv.2018.02.037
- Prasad J, Netam AK, Sahu MK, Satapathy T. Current Concepts in Clinical Based Management of Diabetic Foot Infections: A Review. Res J Pharmacol Pharmacodyn. 2017;9(3):157-166. Available from: http://dx.doi.org/10.5958/2321-5836.2017.00027.1
- Bhushan B. Introduction to nanotechnology. In: Springer Handbook of Nanotechnology. 2017;1-9. Available from: https://link.springer.com/chapter/10.1007/978-3-662-54357-3_1

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
