International Journal of Nanomaterials, Nanotechnology and Nanomedicine
Morphological Analysis of Nanocapsules Processed based on Deer Antler Extract
Sevil Mehraliyeva1*, Farah Madatli1, Sevinj Musayeva1, Betul Ceviz Sakar2 and Zeyneb Orhan2
2Atatürk University Eastern Anadolu High Technologies Application and Research Center (DAYTAM), Erzurum, Turkey
Cite this as
Mehraliyeva S, Musayeva S, Sakar BC, Orhan Z. Morphological Analysis of Nanocapsules Processed based on Deer Antler Extract. Int J Nanomater Nanotechnol Nanomed. 2024;10(2):052-055. Available from: 10.17352/2455-3492.000062Copyright License
© 2024 Mehraliyeva S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.The research work is devoted to the acquisition of nanocapsules, which are one of the drug delivery systems, and their morphological analysis. In modern times, the development of new drug delivery systems from natural raw materials in the treatment and prevention of diseases of various origins is considered a priority issue of pharmacy. From this point of view, the preparation and analysis of nanocapsules based on the extract from the studied deer antlers is of great importance.
As a result of the morphological analysis of nanocapsules prepared from deer antlers grown in the climatic conditions of Azerbaijan, carrageenan-coated nanocapsules have sizes 118.3-243.6 nm in SEM, 10-248 nm in TEM, and tragacanth-coated nanocapsules have sizes 33.15-224.1 nm in SEM, TEM was determined to be 15-254 nm. During numerous studies, it was determined that nanocapsules prepared with tracagant proved to be more intense and more stable. Researches were carried out in the pharmaceutical technology laboratory of Azerbaijan Medical University and AHTARC (DAYTAM) of Atatürk University of the Republic of Turkey. As a result of the conducted research, it was clear that the use of nanocapsules developed by an effective technological method from deer antlers, which has sufficient raw material reserves in Azerbaijan, is considered appropriate for future treatment of diseases in oncology, infertility, osteoarthritis, cardiovascular, and nervous system.
Introduction
One of the important issues facing medical and pharmaceutical science in modern times is the surface coating of extracts from deer antlers, which are rich in enzymes, hormones, vitamins, and peptides. It is known that the new drug forms obtained as a result of surface coating of unstable, bad-smelling, and tasteless medicinal substances are called nanocapsules.
Nanocapsules typically exist in the smallest sizes ranging from 10 nm to 1000 nm. They consist of a liquid/solid core, where the drug is placed in a cavity surrounded by a type of polymer membrane made of natural or synthetic polymers. They have attracted great interest due to their protective coating, which is usually pyrophoric, easily oxidized, and has a prolonged effect on the release of active substances. In such medicinal forms, the medicinal substance in the core is protected from adverse effects of the enviro nment, its stability is ensured, its biological assimilation is increased, and toxic effects are reduced [1-4]. Therefore, the encapsulation of extracts from deer (Cervus elaphus sibiricus) antlers, whose composition is rich in many chemical compounds, is considered one of the urgent issues.
In vitro and in vivo pharmacological studies have shown that the base of deer antler has immunomodulatory, anti-cancer, viral, anti-stress, anti-osteoporosis, anti-inflammatory, anti-infertility, pain-relieving, antibacterial, antioxidant, hypoglycemic, anti-aging effects. Although the mechanism of action is still unclear, pharmacological activity can be mainly attributed to biologically active compounds, amino acids, polypeptides, and proteins. According to animal studies and clinical trials, deer antler base does not cause serious side effects. During rehabilitation after operations, radiation, and chemotherapy, medicinal preparations made on the basis of horns can provide the body with a complex of unique substances and elements useful for normal life [5-8].
In addition to countries like Japan, Vietnam, China, Korea, and Russia, Pant baths are widely used in Azerbaijan as well. This eliminates chronic fatigue syndrome and improves the mental and emotional state of a person. It also increases the intensity of metabolism and metabolic processes in the body and accelerates microcirculation in the vessels. In addition, the treatment of diseases of the genitourinary system, gastrointestinal system, bronchial asthma, osteochondrosis, and a number of skin diseases [1,9].
It was found that the short shelf life of medicines and cosmetics obtained from deer antlers has adverse effects on many diseases (arterial hypertension, atherosclerosis, active form of tuberculosis, severe kidney diseases, diarrhea, brain trauma, and epilepsy), as well as low bioavailability [10].
This leads to a decrease in the possible pharmacotherapeutic effect. Therefore, therapeutic systems are used to ensure more active absorption and action of medicinal substances, which increases absorption. Such drug delivery systems ensure the delivery of the active substance to the damaged area, i.e. the target cell, preventing damage to healthy cells during the treatment, allowing to obtain a high therapeutic effect with smaller doses of the drug. Carrier systems used for the delivery of active substances include microcapsules, microspheres, liposomes, niosomes, nanocapsules, etc. [2,11-13].
Taking into account the above, it was considered appropriate to prepare nanocapsules with a long-term effect based on the extract obtained from deer antlers.
Materials and methods
Firstly, the nanocapsules were obtained from non-ossified deer horns in the laboratory of the Department of Pharmaceutical Technology of Azerbaijan Medical University. Deer antlers from the Altyağac deer farm located in the Khizi region of Azerbaijan were shredded and extracted. Then the nanocapsules were obtained [1,14]. Nanocapsules from deer antlers, and distilled water. The dimensions of the studied nanocapsules were investigated at DAYTAM (Doğu Anadolu Yüksek Teknoloji Uygulama ve Araştırma Merkezi), which operates under Atatürk University in Turkey. SEM analysis of nanocapsules obtained from deer antlers was performed on a Zeiss Sigma 300 device. TEM analysis of nanocapsules was performed on a Hitachi HT-7700 device.
Results and discussion
In performing the SEM analysis of the studied nanocapsules, carbon tubes were attached to the stubs and placed on the samples. Then the stubs were placed on the stage. The stage was placed in the device to be placed in containers and placed in 800s palladium containers. Then the prepared samples were placed in the SEM device. It was kept in the device for 5 minutes to get a vacuum. After that, images were taken at EHT-5 kV.
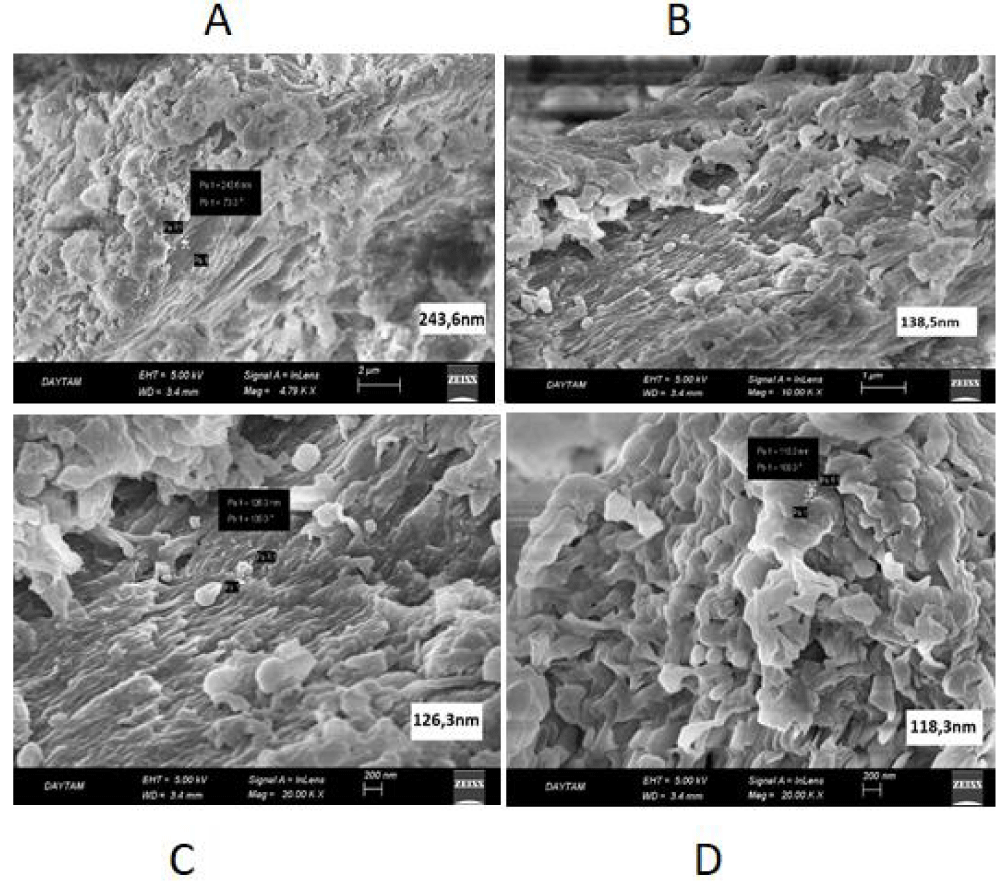
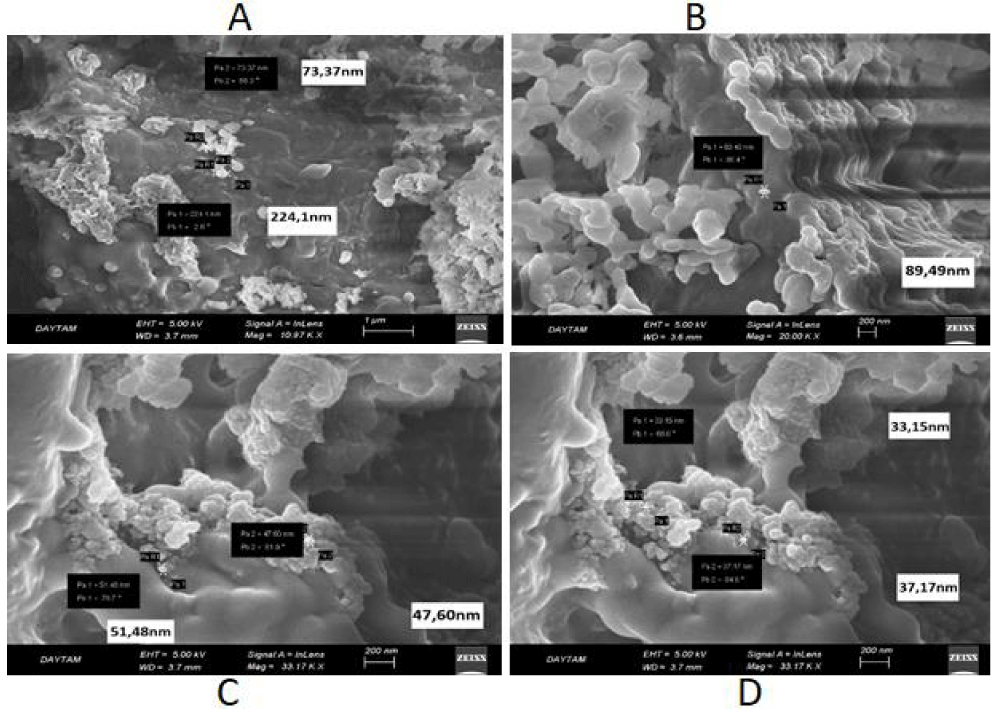
Nanoparticle measurements were performed at different magnifications (Figures 1,2).
As can be seen from Figure 1, the diameter of the nanocapsules measured at 2 µm, 1 µm, and 200 nm scales is 118.3-243.6 nm.
The 224.1 nm diameter of nanocapsules drawn on a scale of 1 µm and 200 nm; 89.49 nm; 73.37 nm; 51.48 nm; 47.60 nm; was determined to be 37.17 nm and 33.15 nm in size.
Performing the TEM analysis, the amount of solid sample in suspension should be between 0.1% and 1% when powdered samples are in Eppendorf tubes with distilled water. The solid sample in suspension was kept in an ultrasonic water bath until completely homogeneously dispersed. Care must be taken to avoid visible balls.
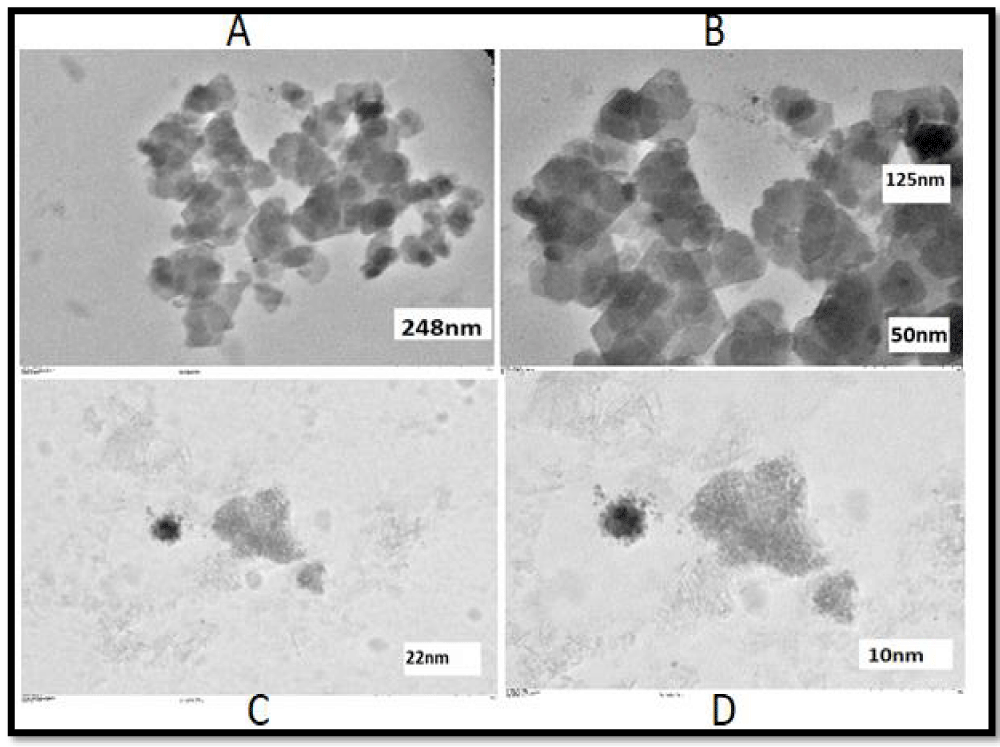
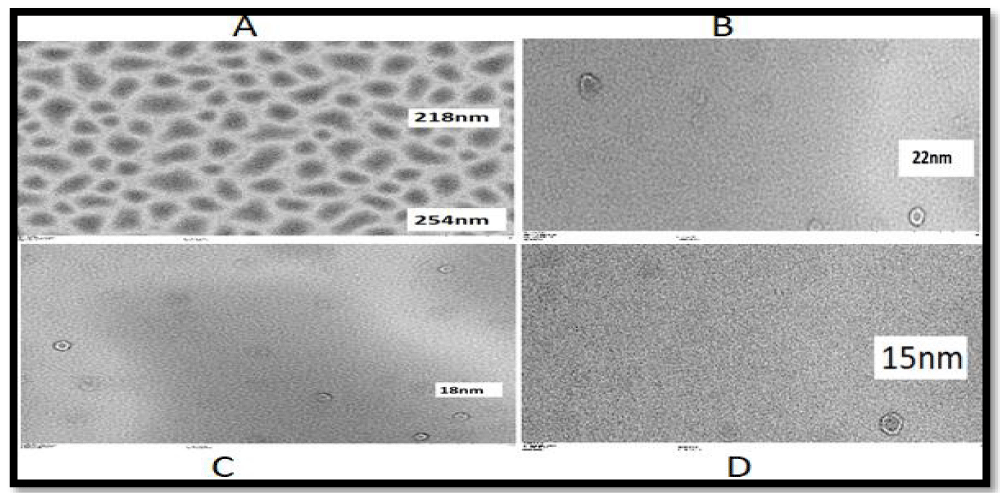
The resulting suspension was dropped (in 3-5 µl) onto a fixed carbon-coated grid with the help of a micropipette. The substance placed on the carbon-coated grill is kept until completely dry. The dried sample was placed in a Hitachi HT-7700 device, a Lanthanum hexaboride (LaB6) electron gun operating under an accelerating voltage in the range of 40-120 kV. With the push of a button, the device can switch from search mode (on-screen camera view) to high-quality, full-size image capture mode (main camera mode), allowing one to quickly capture a selected area. TEM images of the presented samples (carrageenan and tragacanth-coated nanocapsules) are depicted in Figures 3,4.
As can be seen from Figure 3, as a result of TEM analysis 1 µm, 500 nm, 200 nm, and 100 nm scale images were obtained for the carrageenan-coated nanocapsules, the diameter of nanoparticles was found to be 248 nm, 50 nm, 22 nm, and 10 nm, respectively.
As can be seen from Figure 4, tragacanth-coated nanocapsules on the scales of 1µm, 500 nm, 200 nm, and 100 nm as a result of TEM analysis, the diameter of nanoparticles was found to be 254-218 nm, 22 nm, 18 nm, 15 nm, respectively.
As a result of the morphological analysis of the nanocapsules prepared from deer antlers, the SEM analysis of carrageenan-coated nanocapsules was 118.3-243.6 nm, and the TEM analysis was 10-248 nm, and the SEM analysis of the tragacanth-coated nanocapsules was 33.15-224.1 nm; In the TEM analysis, the size of the nanocapsules was determined to be 15-254 nm. Comparative analyses proved that nanocapsules prepared with tragacanth are more intense and more stable. This will allow the nanocapsules to be transported to the pathological site in the treatment of relevant diseases, the drug to bind to the relevant receptors located in the target cells, the drug release rate, and the high pharmacotherapeutic effect.
Conclusion
As a result of the research, it became clear that the use of nanocapsules developed by an efficient technological method from the antlers of deer, which has sufficient raw materials in Azerbaijan, is considered appropriate in the future in the treatment of diseases such as oncology, infertility, osteoarthritis, cardiovascular, and nervous system.
- Mehraliyeva S. Claim document for the invention No. a 2022 0046. Method of obtaining nanocapsules from deer antlers. 2022 Mar 18.
- Delcea M, Yashchenok A, Kristina Videnova, Oliver Kreft, Helmuth Möhwald, Andre G. Skirtach. Multicompartmental Micro- and Nanocapsules: Hierarchy and Applications in Biosciences Macromolecular Bioscience. Micro‐ and Nanocapsules for Biological and Biomedical Applications. 2010;10(5):465-474. doi:10.1002/mabi. 200900359. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/mabi.200900359
- Medvedeva NV, Ipatova OM, Ivanov IuD, Drozhzhin AI, Archakov AI. Nanobiotechnology and nanomedicine. Biomed Khim. 2006;52(6):529-46. Available from: https://pubmed.ncbi.nlm.nih.gov/17288245/
- Kothamasu P, Kanumur H, Ravur N, Maddu C, Parasuramrajam R, Thangavel S. Nanocapsules: the weapons for novel drug delivery systems. Bioimpacts. 2012;2(2):71-81. Available from: https://pubmed.ncbi.nlm.nih.gov/23678444/
- Tansathien K, Suriyaaumporn P, Charoenputtakhun P, Ngawhirunpat T, Opanasopit P, Rangsimawong W. Development of Sponge Microspicule Cream as a Transdermal Delivery System for Protein and Growth Factors from Deer Antler Velvet Extract. Biol Pharm Bull. 2019;42(7):1207-1215. Available from: https://pubmed.ncbi.nlm.nih.gov/31257296/
- Chonco L, Landete-Castillejos T, Serrano-Heras G, Serrano MP, Pérez-Barbería FJ, González-Armesto C, et al. Anti-tumour activity of deer growing antlers and its potential applications in the treatment of malignant gliomas. Sci Rep. 2021;11(1):42. Available from: https://pubmed.ncbi.nlm.nih.gov/33420194/
- Pham TL, Thi TT, Nguyen HT, Lao TD, Binh NT, Nguyen QD. Anti-Aging Effects of a Serum Based on Coconut Oil Combined with Deer Antler Stem Cell Extract on a Mouse Model of Skin Aging. Cells. 2022;11(4):597. Available from: https://pubmed.ncbi.nlm.nih.gov/35203249/
- Zang ZJ, Tang HF, Tuo Y, Xing WJ, Ji SY, Gao Y, et al. Effects of velvet antler polypeptide on sexual behavior and testosterone synthesis in aging male mice. Asian J Androl. 2016;18(4):613-9. Available from: https://pubmed.ncbi.nlm.nih.gov/26608944/
- Zemtsova NP, Turetskova VF, Makarova OG. Determination of amino acids in deer's horns: methodical validation. Pharmacy. Moscow, 2016;38-41.
- Zemtsova NP. Development of technology and standardization of a general tonisuric drug using the basis of Marala antlers marked. Autoref. Dissertation for the degree of candidate of pharmaceutical sciences. 2019;171.
- Tiwari G, Tiwari R, Sriwastawa B, Bhati L, Pandey S, Pandey P, et al. Drug delivery systems: An updated review. Int J Pharm Investig. 2012;2(1):2-11. Available from: https://pubmed.ncbi.nlm.nih.gov/23071954/
- Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, Diaz-Torres LA, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(1):71. Available from: https://pubmed.ncbi.nlm.nih.gov/30231877/
- Yunxin X, Angel T, Alexander W Jackson, Ben J Boyd. Nonspherical Nanocapsules as Long-Circulating Drug Delivery Systems. Chem Mater. 2022;34(6):2503–2530. Available from: https://pubs.acs.org/doi/10.1021/acs.chemmater.1c03573
- Mehmet Atesh. Measurement and Analysis Techniques of Nanoparticles. Turkish Journal of Scientific Reviews. 2018;11(1):63-69. Available from: https://dergipark.org.tr/en/download/article-file/608610

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
