International Journal of Nanomaterials, Nanotechnology and Nanomedicine
Scaffold-based microsphere in drug delivery system
Girja Sharma*, PK Sharma and Md Aftab Alam
Cite this as
Sharma G, Sharma PK, Alam MA (2024) Scaffold-based microsphere in drug delivery system. Int J Nanomater Nanotechnol Nanomed 10(1): 016-022. DOI: 10.17352/2455-3492.000058Copyright License
© 2024 Sharma G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Microspheres are free-flowing powders having a synthetic and natural polymer. A targeted drug delivery system can overcome some of the problems of conventional therapy and enhance the therapeutic efficacy of the drug. This is a biodegradable and non-biodegradable efficacy of a given drug. There are various approaches to delivering a therapeutic substance to the target site in a sustained controlled release fashion. One approach is a scaffold-based microsphere for drug delivery, where the target site is very specific if modified, and the proper concentration is maintained at the site of interest without causing toxic effects. Microspheres received much attention not only for control release but also in drug targeting for example in anticancer therapy. Moreover, the joining of medications (i.e., incendiary inhibitors and additionally anti-microbial) into platforms might be utilized to forestall contamination after medical procedures and other infections for the longer term. The framework additionally can be utilized to give sufficient signs to the cells, to initiate and keep them in their coveted separation organization, and to keep up their survival and development. The present survey gives an itemized record of the requirement for the advancement of frameworks alongside the materials utilized and systems embraced to fabricate platforms for tissue designing and delay. The present review gives a detailed account of the need for the development of scaffolds along with the materials used and techniques adopted to manufacture scaffolds for tissue engineering and microspheres for drug delivery systems.
Introduction
The targeted delivery system has been prepared in a way that it delivers the drug at a pre-determined rate and for a predetermined period of time [1,2]. Microspheres are spherical in shape with a diameter range of (1-1000 μm), and sometimes microspheres are also referred to as microparticles. It is manufactured by different types of natural and synthetic materials [3]. Then the microspheres release the drug in the manner of control rate and overcome the problem encountered by conventional drug delivery systems, thus increasing the therapeutic efficacy of the drug [4]. The main purpose of this system is to fix the optimum plasma drug concentration to increase the efficacy, safety, and bioavailability of the drug [5]. The reason behind this development of the delivery system for microspheres is to make a therapeutic agent do their best when the drug is administered into the body. This means maximum efficacy and minimal toxicity leading to the successful application of many therapeutic agents [6]. This type of approach allows the exact small quantity of potent drugs to be delivered. Consequently, the decrease in the drug concentration at the site and the target site then the protection of the labile compound before and after the administration of the drug at the site of action [7,8]. Different types of variety used as drug carriers, include the immune globulins serum of proteins, microspheres, and even cells such as the erythrocytes [9].
Microspheres
These are micrometric reservoir systems. They differ from microparticles by having a drug that is located under the polymeric shell [10,11]. Release control by the dissolution method by another method called diffusion method, or both [12]. Microspheres are very strict in filling spherical empty debris without a center, those microspheres are commonly unfastened flowing powders it is miles inclusive of proteins or artificial polymers, strong biodegradable microspheres disperse the drug or dissolve all through particle matrix and have the ability for controlled release of drugs 3magnetic microspheres used for the goal of tumors [13].
Scaffolds
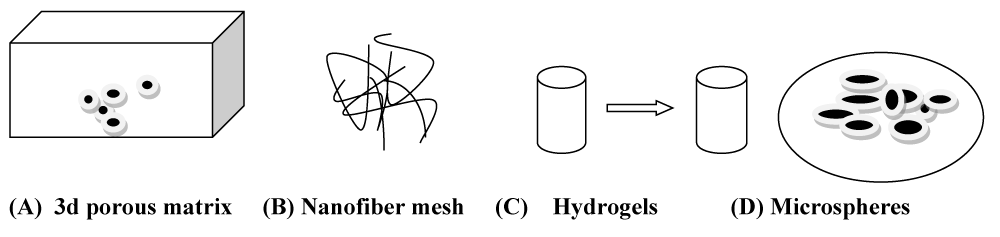
These are porous biomaterial scaffolds and are the biomaterials found in two types of materials; natural and synthetic polymers. Scaffolds are used for tissue regeneration [14,15]. Scaffolds are basically implants and injects used to deliver cells, drugs, and genes into the body. Different types of forms of polymeric scaffold delivery are available for cell and drug delivery (Figure 1). It is a three-dimensional porous matrix and fibrous matrix, thermo-sensitive sol-gel transition hydrogel, and Porous Microsphere. Scaffolds provide a suitable substrate for cell and tissue attachments. Nowadays many types of clinical procedures are performed to replace and repair the tissue in the human body [16].
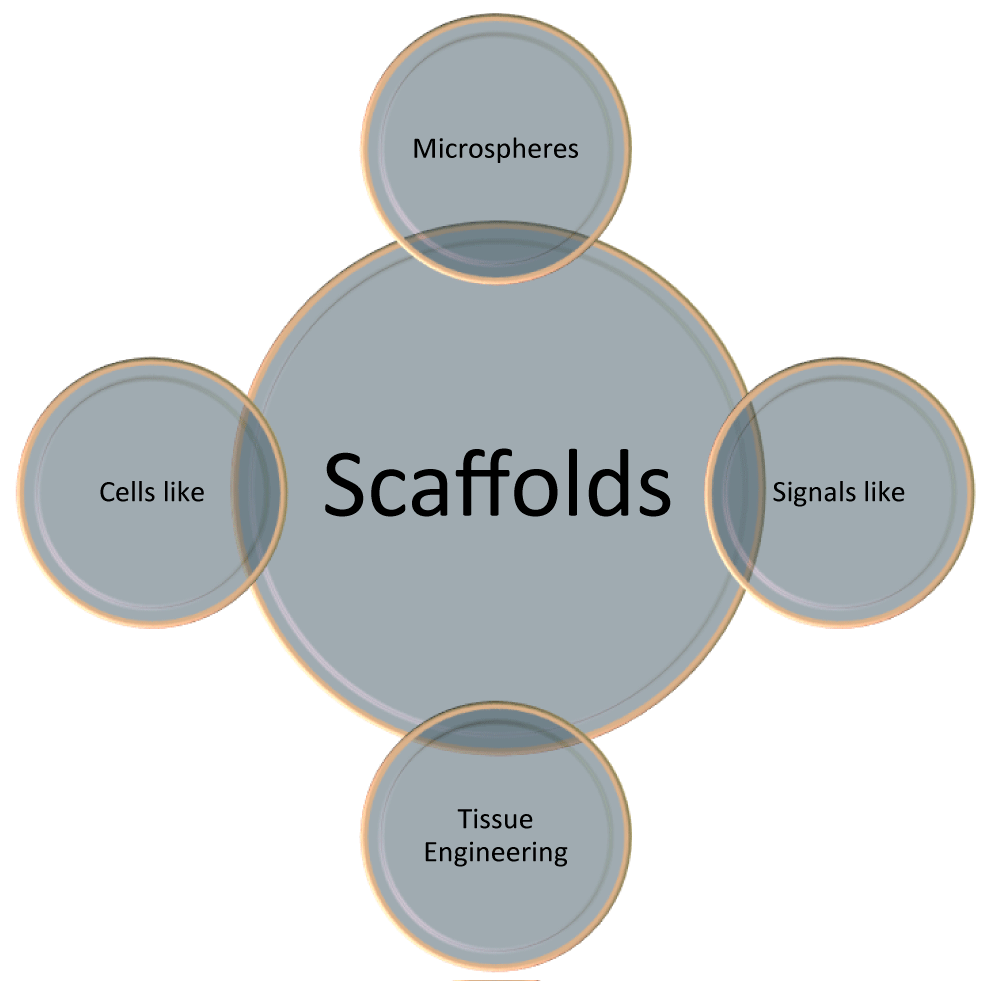
This type of scaffold technology is based on the positive interaction between three components;
- Scaffolds that hold every cell together to create the physical formation of tissue.
- Cells create the tissue.
- Biological factors their growth value (Figure 2) [17].
Scaffolds for cell delivery should possess the following characteristics:
- Mechanical property is complete to shield cells from a tensile force without biomechanical cues.
- Shape, Size, Growth, Their stamina.
- Compatibility.
- Mechanical Strength [18].
- Bio adsorption at a predetermined period of time.
- Biocompatible chemical ingredients and degradation products, causing inflammatory responses [19].
- Stability in their ability to release the drug at a predetermined rate.
These are the challenges in the creativity and manufacturing of scaffolds as they possess the above requirements and their ability to control the release of kinetic drugs and tissue regeneration [20].
Biomaterials used for scaffold preparations
Natural polymer: Different Natural polymers i.e., alginate, proteins, albumins, cellulose, starch, heparin. They are also used for cell and gene deliveries. The merits of natural polymers are biocompatibility, stability, and easy processing. They all more closely mimic the Extracellular Matrix (ECM) and remove the cross-contamination.
Synthetic polymers: This type of synthetic polymer is divided into two parts: biodegradable and non-biodegradable. It is a polyvinyl alcohol [21].
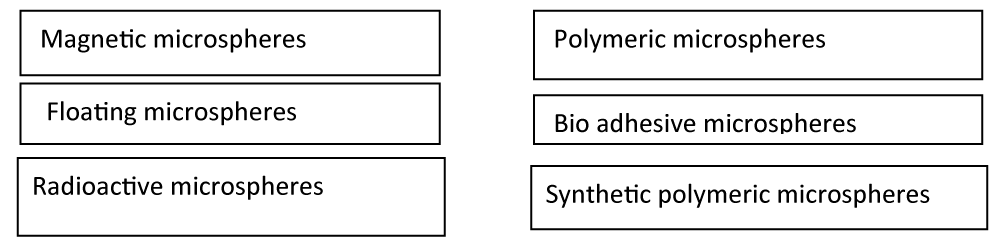
Types of microspheres: The various types of Microspheres are as follows: (Figure 3)
Magnetic microspheres: These are the microspheres having super molecular particles with a small sphere circulating in the capillaries without any embolic occlusion. However, it has a sufficient amount of ferromagnetic property to be captured and dragged into the tissue. That is one example of the specific targeting by magnetic microspheres and polymers like chitosan and dextran applied to the magnetic field [22].
Floating microspheres: This type of microsphere has a lower bulk density compared to gastric fluid and it remains in the stomach without affecting the rate of the gastric emptying fluid. The drugs will be released slowly at the targeting site. Then the systems float on the gastric content which increases the gastric toxicity and increases their plasma concentration. Have a high chance to reduce the dose dumping [23].
Radioactive microspheres: These types of microspheres are injected into the arteries that directly target the tumor. So these types of conditions of radioactive microspheres deliver the high radio radiation dose to different types of targeted areas without any damaging normal suspended. Radio-labeled blood cells are also used nowadays. The pre-formulation of radioactive microspheres having different types of emitters is easy to use and never requires time-wasting labeling procedures. The radioactive microspheres' homogenous number is made of polystyrene and is not biodegradable. Then they may be inappropriate for clinical use [24].
Polymeric microspheres are the microsphere divided into two types:
Biodegradable polymeric microspheres: These are the natural polymers when starch is used because they are biodegradable and biocompatible, also they are bio-adhesive in nature. Biodegradable polymers have a rate and extent of the drug release controlled by the concentration of the polymer like Hydroxypropyl Methylcellulose (HPMC) and release pattern in a sustained manner. The main disadvantage of this is clinical use of drug loading efficiency of biodegradable microspheres is very high. They difficult to control the drug release [25].
Synthetic polymeric microspheres: These types of microspheres are used in clinical applications and also used as bulking agents, drug delivery vehicles proved to be safe and biocompatible [26]. Many types of sustained-release drugs or in the preparation for anti-malarial drugs as well are formulated by using co-polymers like polylactic acid and polyglycolic acid [27].
Bio-adhesive microspheres: These types of adhesion are defined as a sticky form of drug to the membrane by using of sticky category of the water-soluble polymer. Basically, the term bio-adhesion means that the material binds to biological substrates, like a mucus membrane. Adhesion of the drug delivery devices to mucosal tissue gives the possibility of creating intimate contact at the site of targeting administration. This combination of the controlled release of the drug improved patient compliance by reducing the frequency of the administration [28].
Advantages of microspheres
Particle size is less of a value for increasing the solubility of one feet soluble drugs [29]. Treatment of gastric intestinal disorders such as gastro-esophageal reflux [30], offers a perfect route, for systemic delivery of the drugs. Which highly passed the first pass metabolism and gives the chance of high bioavailability [31]. Drugs that have less stability in an acidic environment are destroyed by the enzymatic environment of the intestine and can be administered by this route example: Buckle, sublingual [32].
Limitations
Controlled and protected are all the mentioned microspheres and these have different preservation from the one to other dosage form [33]. The cost of the ingredients and their processing of the preparation, are high than the human formulations. The bunch of polymer matrices has an impact on the environment. The polymer additives include plasticizers, stabilizers, antioxidants, and fillers. They have less reproducibility. The environment is full of steam for the loss of products of their polymer matrix produced by force to light, hydrolysis, oxidation, and biological agents [34]. So, the controlled release rate of the microspheres may fluctuate on certain factors like extrinsic factors like food, rate of transit through gut over rate, etc [35].
Method of preparation
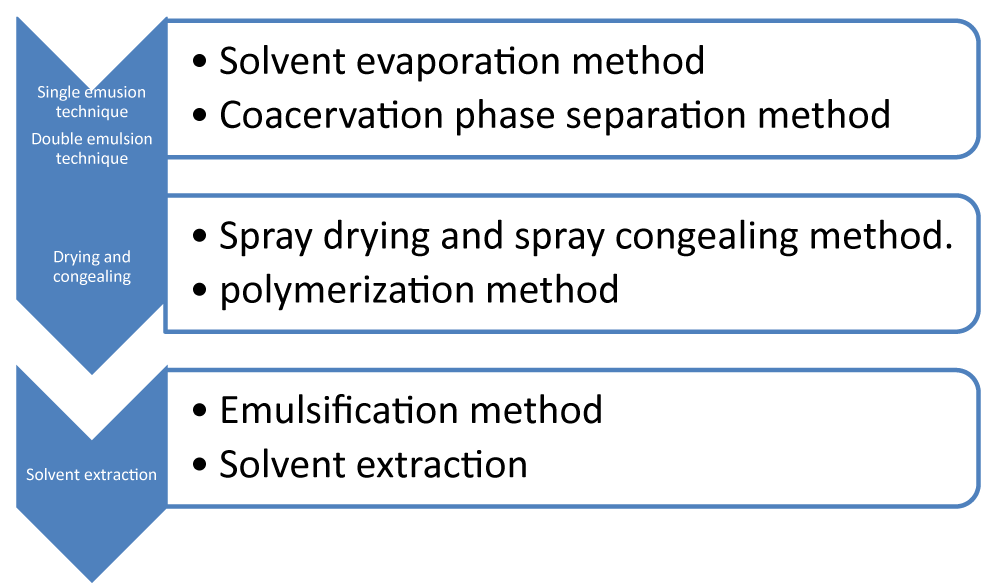
There are several types of methods to prepare microspheres (Figure 4).
Solvent evaporation method
These are the evaporation method divided in two parts
Single emulsion technique: In this method, the natural polymer microspheres are prepared by this technique. Firstly, the polymer and drug are dissolved in an aqueous media followed by an organic medium like oil, and globules, after this, globules are cross-linked by heat or by using the chemical cross-linkers. The chemicals used are formaldehyde and glutaraldehyde [36]. Many types of carbohydrates and proteins are mainly prepared by that technique. Add dispersion into the heated oil. However, this method is not perfect for the thermo-labile drugs.
By chemical cross-linking agent: Chemical cross-linkers suffer the demerits of the pure form of the polymer. In the case of the heating and suspension or a combined manner of the monomers of the active drugs as droplets in the continuous phase. It is also known as pearl polymerization. In the case of emulsion polymerization, they have an initiator present in an aqueous phase [37,38].
Double emulsion technique: This is the method that can be used with the two types of polymer either natural or synthetic polymer and is more comfortable for water-soluble drugs, like peptides, proteins, and vaccines [39]. This type of method has various formations of multiple emulsions like o/w/o. The aqueous protein solution dissolved in the lipophilic organic phase. The protein solution has an active phase. These are the phases of the polymer solution that is capsulated of the protein of the aqueous phase [40]. The primary emulsion of the sonication method before adding the aqueous solution of the polyvinyl alcohol (PVA). The result of this formation of double emulsion. It is known by the solvent evaporation and the solvent extraction process [41]. This method is carried out by maintaining the emulsion on reduced pressure and also by stirring the emulsion after this the organic phase is carried out and evaporates properly [42]. After this emulsion is added to a large quantity of water this organic phase diffuses out. The solid particles of the microspheres are continuously obtained by filtration and washed with N-Hexane and acetone or other organic solvent to separate out the traces of the oil form from the surface area [43].
Coacervation phase separation method: This method's main purpose is to prepare the reservoir type of the system. This type of method is used to encapsulate water-soluble drugs example: peptides, proteins, and some other matrix, when a drug is hydrophobic in nature [44]. This is the main principle of decreasing the solubility of the polymer to the organic phase. The drug particle is properly dissolved in the solution of the polymer and the incompatibility of this polymer adds to make the polymer phase separation and engulf the drug size. Add the non-solvent result to solidify the polymer [45].
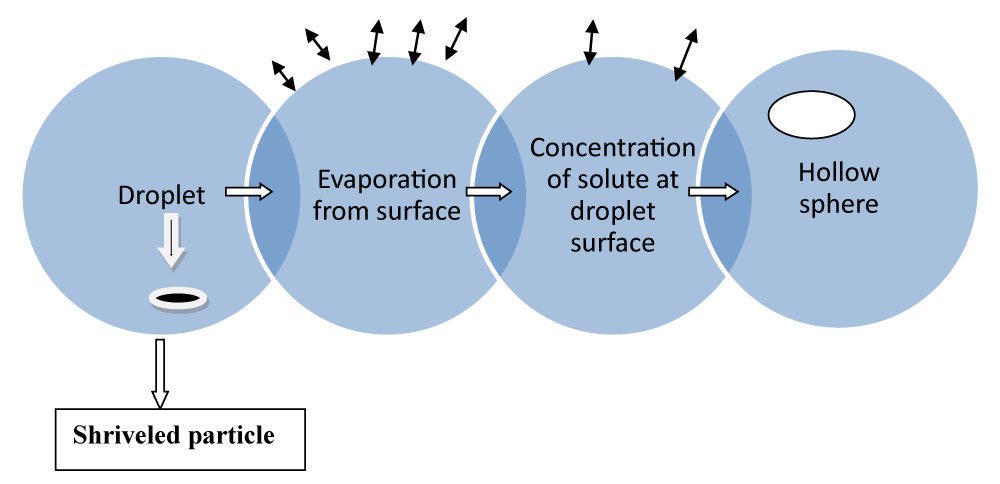
Spray drying and spray congealing method: In this method, firstly, the polymer is dissolved in a perfectly volatile organic solvent like dichloromethane, or acetone (Figure 5). The method of spray drying process is used to encapsulate various like penicillin [46].
Polymerization method: It is the new method for the encapsulation form of the protective microcapsule capsule coatings in situ. It is between the core material substance and the continuous phase which is studied formulation and evaluation by sustained release microsphere acetazolamide solvent evaporation method using RS and RL (acrylic polymers) as polymer and found the particle size of microcapsules were influenced by the concentration stirring speed [47]. This method has two types of polymerization:
Normal polymerization: This polymerization is a monomer and a mixture of different numbers of monomers with the initiator usually heated to this polymerization. These polymers are obtained by the molded, as microspheres. The drug loading of hot air has small drops, the fine mist, and the continuous solvent evaporates leading to the form of the microspheres. The size of the microspheres range is 1-100 μm. Then the hot air separation of the microparticle means that the cyclone separator when the traces of the solvent are removed by vacuum drying. The main perfection of the process is good feasibility of operation. This is the method is very useful [48].
Interfacial polymerization: It involves the reaction of different types of monomers at the interfaces between the two types of immiscible liquid phases to form a film of the polymer that is the very creativity of the dispersed phase. Another part of this technique has two reacting monomers employed: first dissolved in the aqueous phase and while other is dispersed in the continuous phase.
Emulsification method: This type of emulsification method is formed by heating the aqueous type of drug solution and it can be dispersed in molted wax because it forms a w/o emulsion, which is emulsification by heating the external phase to form a w/o/w emulsion [49].
Solvent extraction method: This method is used for the manufacture of microparticles; they have removal of the removal phase by extraction. Under this method, water-miscible organic solvents are used, such as isopropanol. The organic phase can then be separated by the extraction with water [50,51].
Applications of microspheres in drug delivery
Some of the pharmaceutical applications are discussed as follows:
Ophthalmic drug delivery: This polymer exhibits physic-chemical biological behavior like a bio-adhesion, its permeability enhances properties and their interest physic chemical characteristics, which makes a unique or different kind of material for the perfect design of the ocular drug delivery. Due to their elastic properties, they can be used for ophthalmic delivery such as ointments, and chitosan gels, and improve their adhesion to musing, which is coated by the conjunctiva and the corneal surface area of the eye and increase their procorneal drug for the different periods of times. Then the drug shows elimination by the continuous flow [52].
In vaccine delivery: The pre-replacement of a vaccine against the microbes and the toxic product. Biodegradable drug delivery systems for vaccines that are given by parental route may resolve the conventional vaccines. Several types of vaccines have been encapsulated in biodegradable polymer microspheres, including the different types of vaccines [53].
Targeting drug delivery: The meaning of targeting like the site of a specific drug is well perfect dogma, which is a main focus of research and treatment. The efficacy of the drug release depends on their interaction with the receptors [54].
Buccal absorption test: This method is perfect for measuring the extent of the drug loss by the human oral type cavity for the single and the different components of the drugs. This test was successfully used to find out the relative importance of the drug components, their time, the drug concentration, and their pH solution while the drug is attached to the oral cavity [55]. Release of the proteins and the hormones over a long period of time. The passive target of the leaky tumor is the vessel then the tumor cells, by the intravenous application [56].
Drug polymer binding: The policy of the hold microspheres by matrix bounces chemicals. The installation of this hydrophobic and electrostatic interaction is managed by their existence [57]. Drugs are examples like peptides and proteins that also are targeted by this system. Also, the diagnostic microspheres are used for liver metastases and also the structure by supra magnetic iron oxides [58]. The coated microspheres have many possible applications in the science of matter and substance research. The Polythene microspheres are the spacers used in the LCD screens [59]. Microspheres made by the biocompatible polymer can be added as cell microcarriers by other cells [60].
Ceramics and bioglasses: Bio glasses are an important class of material in bone regeneration. These compounds, having an inorganic composition with a unique one changed into compounds based on Na2O-CaO-SiO2-P2O5 [61]. These are capable of integration into the bone after they are reabsorbed, and the bone is increased without dissolving [62].
Hydro gel-based system: Hydrogel matrices are physically and chemically passed, and the polymers of water-soluble, which are the swell to shape a gel-based substance on the subjection to the water [63]. Hydrogels appeal to organic applications due to their high content of water and their biocompatibility [64].
Application of microspheres in pharmaceutical industries
This is for taste and odor masking, as well as delaying the volatilization. Then the separation of the incompatible substances, improvement of the flow properties of powders, and increasing the stability of the drugs again, the outer conditions. Also, the safe handling of toxic substances by incorporating dispersed such as material in the aqueous medium [65].
Microspheres recent updates and advancements, Patent
One of the recent updates of the microspheres for a carrier for drugs are the very fine particles and consist of protein and synthetic polymer that are biodegradable in nature then have a perfect particle size of less than 200μ [66,67]. Some recently updated patents are shown in Table 1.
Conclusion
Scaffolds had been investigated with respect to the fabric requirement, property, and technology for the production of scaffolds. The world of biomaterials has fulfilled an important function in the development of tissue-engineered merchandise. No matter this, a few scaffolds are to be had commercially, mainly for cell/drug transport. A maximum of the scaffolds studied are nevertheless within the studies diploma and are however to be authorized for medical use. Looking into comfort and practicability, there is gigantic scope in growing injectable gel scaffolds due to the fact that they are smooth to use, bendy, and incorporate using at-ease adjutants; many of them are already indexed within the typically diagnosed as safe listing or actually have been permitted by the food and drug management. New biodegradable polymers want to be developed to fulfill all requirements for surgically implantable scaffolds.
- Banker GS, Rhodes CT, editors. Modern pharmaceutics. 4th ed. New York: Marcel Dekker Inc; 2002; 502-527.
- Popli H, Sharma SN. Trends in oral sustained-release formulations-I. The Eastern Pharmacist. 1996; 32:99-103.
- Riz Kalla. Microspheres drug delivery system. 2006; 3:15-30.
- Srivastava P, Visht S. Application and advancement of microspheres as controlled drug delivery system. International Journal of Pharmacy & Life Sciences. 2013; 4:2583-2594.
- Jha MK. Modified release formulations to achieve the quality target product profile (QTPP). International Journal of Pharmaceutical Sciences and Research. 2012; 3:2376-2386.
- Vyas SP, Khar RK. Targeted and Controlled drug delivery. 7th ed. 2013; 102-107:418.
- Burgress DJ, Hickey AJ. The University of North Carolina at Chapel Hill, Carolina, U.S.A, Encyclopedia of Pharmaceutical Technology. 1997; 2328-2337.
- Jain NK. Controlled and Novel drug delivery. 4th ed. 2001; 236-237:21.
- Vyas SP, Khar RK. Targeted and Controlled drug delivery. 7th ed. 1999; 418.
- Komatsu M, Tagawa K, Kawata M, Goto S. Biopharmaceutical evaluation of gelatin microcapsules of sulfonamides. Chem Pharm Bull (Tokyo). 1983 Jan;31(1):262-8. doi: 10.1248/cpb.31.262. PMID: 6850940.
- Li SP, Kowalski CR, Feld KM, Grim WM. Recent Advances in Microencapsulation Technology and Equipment. Drug Delivery Ind Pharm. 1988; 14:353-376.
- Gupta PK, Hung CT. J Pharm Sci. 1998;78:745.
- Prasad SGB, Gupta VRM, Devanna N, Jayasurya K. Microspheres as drug delivery system – A review. JGTPS. 2014.
- Langer R, Vacanti JP. Tissue engineering. Science. 1993 May 14;260(5110):920-6. doi: 10.1126/science.8493529. PMID: 8493529.
- Griffith LG, Naughton G. Tissue engineering--current challenges and expanding opportunities. Science. 2002 Feb 8;295(5557):1009-14. doi: 10.1126/science.1069210. PMID: 11834815.
- Langer R. Biomaterials in drug delivery and tissue engineering: one laboratory's experience. Acc Chem Res. 2000 Feb;33(2):94-101. doi: 10.1021/ar9800993. PMID: 10673317.
- Lyons F, Partap S, O'Brien FJ. Part 1: scaffolds and surfaces. Technol Health Care. 2008;16(4):305-17. PMID: 18776607.
- Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000 Dec;21(24):2529-43. doi: 10.1016/s0142-9612(00)00121-6. PMID: 11071603.
- Peter SJ, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Polymer concepts in tissue engineering. J Biomed Mater Res. 1998 Winter;43(4):422-7. doi: 10.1002/(sici)1097-4636(199824)43:4<422::aid-jbm9>3.0.co;2-1. PMID: 9855200.
- Sokolsky-Papkov M, Agashi K, Olaye A, Shakesheff K, Domb AJ. Polymer carriers for drug delivery in tissue engineering. Adv Drug Deliv Rev. 2007 May 30;59(4-5):187-206. doi: 10.1016/j.addr.2007.04.001. Epub 2007 Apr 27. PMID: 17540473.
- Mark Saltzman W, Baldwin SP. Materials for protein delivery in tissue engineering. Adv Drug Deliv Rev. 1998 Aug 3;33(1-2):71-86. doi: 10.1016/s0169-409x(98)00021-0. PMID: 10837654.
- Seng CH. J. Pharm. Sci. 1995; 74 (4): 399-405.
- Lachman LA, Liberman HA. and Kanig JL. The Theory and Practice of Industrial Pharmacy. 3rd edition Varghese Publishing House Mumbai, India. 1991; 414-415.
- Marcus ML, Heistad DD, Ehrhardt JC, Abboud FM. Total and regional cerebral blood flow measurement with 7-10-, 15-, 25-, and 50-mum microspheres. J Appl Physiol. 1976 Apr;40(4):501-7. doi: 10.1152/jappl.1976.40.4.501. PMID: 931870.
- Cleland JL, Duenas ET, Park A, Daugherty A, Kahn J, Kowalski J, Cuthbertson A. Development of poly-(D,L-lactide--coglycolide) microsphere formulations containing recombinant human vascular endothelial growth factor to promote local angiogenesis. J Control Release. 2001 May 14;72(1-3):13-24. doi: 10.1016/s0168-3659(01)00258-9. PMID: 11389981.
- Amsden BG, Goosen M. An examination of the factors affecting the size, distribution, and release characteristics of polymer microbeads made using electrostatics. J Control Rel. 1997; 43:183–196.
- Rojas J, Pinto-Alphandary H, Leo E, Pecquet S, Couvreur P, Gulik A, Fattal E. A polysorbate-based non-ionic surfactant can modulate loading and release of beta-lactoglobulin entrapped in multiphase poly(DL-lactide-co-glycolide) microspheres. Pharm Res. 1999 Feb;16(2):255-60. doi: 10.1023/a:1018880409254. PMID: 10100311.
- Patel J. Bio-adhesion is a topic of current interest in the design of controlled or targeted drug delivery system. 2001.
- Jamini M, Rawat S. A review on microsphere, Res. j. pharm. boil. chem. sci. 2013.
- Dave BS, Amin AF, Patel MM. Gastroretentive drug delivery system of ranitidine hydrochloride: formulation and in vitro evaluation. AAPS PharmSciTech. 2004 Apr 8;5(2):e34. doi: 10.1208/pt050234. PMID: 15760092; PMCID: PMC2750469.
- Punitha S, Girish Y. Polymers in mucoadhesive buccal drug delivery system. International Journal of Research and Pharmaceutical Sciences. 2010; 1: 2;170-186.
- Sachan NK, Bhattacharya A. Basic and Therapeutic Potential of Oral MucoadhesiveMicroparticulate Drug Delivery Systems. International Journal of Pharmaceutical and Clinical Research. 2009; 1: 10-14.
- Kunchu K, Ashwani RV. Albumin microspheres: An Unique System as drug Delivery Carriers for non steroidal anti-inflammatory drugs (NSAIDS). International Journal of Pharmaceutical Sciences Review and Research. 2010; 5:10-17.
- Alpar HO, Field WN, Hyde R, Lewis DA. The transport of microspheres from the gastro-intestinal tract to inflammatory air pouches in the rat. J Pharm Pharmacol. 1989 Mar;41(3):194-6. doi: 10.1111/j.2042-7158.1989.tb06429.x. PMID: 2568449.
- Kunchu K, Ashwani RV. Albumin microspheres: An Unique System as drug Delivery Carriers for non steroidal anti-inflammatory drugs (NSAIDS). International Journal of Pharmaceutical Sciences Review and Research. 2010; 5:10-17.
- Alagusundaram M, Chetty MS, Umashankari K, Badarinath AV, Lavanya C, Ramkanth S. Microspheres as a novel drug delivery system: A review. Int J Chem Tech Res. 2009;1:3: 526- 534.
- Kuriokase AB, Sathireddy P. and Padma Priya S. A review on microspheres. Global Journal of Pharmacology. 2015; 9:28-29.
- Khar RK, Vyas SP. Targeted and Controlled Drug Delivery – Novel Carrier System. New Delhi, CBS Publication and Distributors. 2002.
- Kunchu K, Ashwani RV. An Unique System as drug Delivery Carriers for non steroidal anti-inflammatory drugs (NSAIDS). International Journal of Pharmaceutical Sciences Review and Research. 2010; 5:10-17.
- Vyas and Khar. Targeted and Controlled drug delivery CBS Publishers and distributors. 2001.
- Patel NR, Patel DA, Bharadia PD, Pandya V, Modi V. Microsphere as a novel drug deliver, Int J of Pharm & Life Sci. 2011; 2:8;992- 997.
- PrasanthVV, Moy AC, Mathew ST, Mathapan R. Microspheres: an overview, Int J of Pharm & Biomedical Science. 2011; 2:2; 332-338.
- Jain D, Panda AK, Majumdar DK. Eudragit S100 entrapped insulin microspheres for oral delivery. AAPS PharmSciTech. 2005 Sep 20;6(1):E100-7. doi: 10.1208/pt060116. PMID: 16353953; PMCID: PMC2750417.
- Sunitha S, Amareshwar P, Santhosh KM, Chakravarti P. Preparation and Evaluation of Tramadol Hydrochloride microspheres by phase separation coacervation technique using various solvents and nonsolvents, J of Global Pharm Tech. 2011; 3:4; 33-41.
- Sapkale H, Sorate H, Jagtap N, Abhirao S. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2013; 4:1273-1293.
- Koff. US patent. 1963; 3:080;292.
- Chowdar, DanaSB. Preparation and Evaluation of Ethylcellulose Coated Microcapsules for Controlled Release of Diclofenac. Research Journal of Pharmaceutical, Biological and Chemical Sciences. 2012; ISSN: 0975- 8585.
- Jayaprakash S, Halith SM, Mohamed Firthouse PU, Kulaturanpillai K, Abhijith, Nagarajan M. Preparation and evaluation of biodegradable microspheres of methotrexate. Asian J Pharm. 2009; 3:26-9.
- Lim ST, Martin GP, Berry DJ, Brown MB. Preparation and evaluation of the in vitro drug release properties and mucoadhesion of novel microspheres of hyaluronic acid and chitosan. J Control Release. 2000 May 15;66(2-3):281-92. doi: 10.1016/s0168-3659(99)00285-0. PMID: 10742587.
- Patel NR, Patel DA, Bharadia PD, Pandya V, Modi D. Microsphere as a novel drug delivery, Int. j. pharm. life science. 2011; 2:8:992-7.
- Thummar AV, Kyada CR, Kalyanvat R, Shreevastva B. A review on mucoadhesive microspheres as a novel drug delivery system, International Journal for Pharmaceutical Research Scholars. 2013; 2:2:188-200.
- Kalyan Shweta Sharma, Parmod Kumar. Recent Advancement in Chitosan best Formulation and its Pharmaceutical Application. Pelagia Research Library. 2010; 3:3: 195-210.
- Lin CY, Lin SJ, Yang YC, Wang DY, Cheng HF, Yeh MK. Biodegradable polymeric microsphere-based vaccines and their applications in infectious diseases. Hum Vaccin Immunother. 2015;11(3):650-6. doi: 10.1080/21645515.2015.1009345. PMID: 25839217; PMCID: PMC4514183.
- Khan MS and Doharey V. A review on Nasal Microspheres. International Journal of Pharma Sciences. 2014; 4:496-506.
- Chinna Reddy P, Chaitanya KS, Madhusudan Rao Y. A review on bioadhesive buccal drug delivery systems: current status of formulation and evaluation methods. Daru. 2011;19(6):385-403. PMID: 23008684; PMCID: PMC3436075.
- Shanthi NC, Gupta R and Mahato KA. Traditional and Emerging Applications of Microspheres: A Review International Journal of Pharm Tech Research. 2010; 2:675681.
- Wulff G, Poll HG. Enzyme-analog built polymers. 23 Influence of the structure of the binding sites on the selectivity for racemic resolution, Makromol. Chemistry. 1987; 188:741-748.
- Martin, Philadelphia PA, Lippincott Williams and Wilkins. Physical pharmacy and pharmaceutical sciences, 6th edition. 2011; 442-468.
- Arshady R. Microspheres for biomedical applications: preparation of reactive and labelled microspheres. Biomaterials. 1993;14(1):5-15. doi: 10.1016/0142-9612(93)90015-t. PMID: 7678756.
- Wong DJ, Chang HY. Skin tissue engineering. 2009 Mar 31. In: StemBook [Internet]. Cambridge (MA): Harvard Stem Cell Institute; 2008–. PMID: 20614591.
- Bouler JM, Pilet P, Gauthier O, Verron E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017 Apr 15; 53:1-12. doi: 10.1016/j.actbio.2017.01.076. Epub 2017 Jan 31. PMID: 28159720.
- Hench LL, J Biomed, Mater Res. Bioactivematerials: The potential for tissue regeneration. 1998; 41: 511–518.
- Drury JL, Mooney DJ. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003 Nov;24(24):4337-51. doi: 10.1016/s0142-9612(03)00340-5. PMID: 12922147.
- Hoffman AS. Hydrogels for biomedical applications. Adv Drug Deliv Rev. 2002 Jan 17;54(1):3-12. doi: 10.1016/s0169-409x(01)00239-3. PMID: 11755703.
- Diane J. Burgress, Anthony JHickey. The University of North Carolina at Chapel Hill, Carolina, U.S.A, Encyclopedia of Pharmaceutical Technology. 1997; 2328 -2337.
- Venkatesan K, Manavalan R, Valliapan K. Microencapsulation: a vital technique in novel drug delivery system. J Pharm Sci Res. 2009; 1:4: 26-35.
- Vyas SP, Khar RK. Targeted and Controlled drug delivery. 2009; 07: 418.
- Sciascia ZL, Wei NS. CN 201110142359, Publication. 2011; 28-09-11.
- Ho XG, Danfeng YCLC. CN 201110313846, Publication. 2013; 03-04-13.
- jiao HB, door Jane, Jianjun RX. CN 201210025085, Publication. 2013; 20-03-13.
- Herrero R, Refojo VMF. US08455091, Publication. 1998; 17-02-98.
- Univ LIH. Quebec à Montreal PING. EP19980924438, Publication. 2004; 14-07-04.
- KeeSah H. EP20070808011, Publication. 2013; 03-04-13.
- Ramtoola Z. CA2217462, Publication. 2010; 03-08-10.
- Lewis AL, Maria PWS, Gonzalez-Fajardo VYT. CA2579533, Publication. 2005; 06-09-05.
- Cantor AS, OkChoi H, Delgado JU, KOThu-Van CT. DE1999609777, Publication. 2004; 15-04-04.
- Chen Y, Gray BN. DE1994632867, Publication. 2004; 22-04-04.
- Kavita K, Raje V. Albumin Microspheres: A Unique system as drug delivery carriers for nonsteroidal anti-inflammatory drugs. 2010; 5:2:12.

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
