Archive of Biochemistry
Lesser Weeks of Gestation Correlated with Levels of Cortisol in Ghanaian Women with Preeclampsia, Case-control Study
Grace K Ababio1*, Kwame Adu-Bonsaffoh2, Emmanuel Abindau3 and Isaac Kweku E Quaye4
1Department of Medical Biochemistry, University of Ghana Medical School, Accra, Ghana
2Department of Obstetrics and Gynecology, Korle-Bu Teaching Hospital, Accra, Ghana
3Medical Laboratory Science Department, Accra Technical University, Accra, Ghana
4Department of Medical Biochemistry, University of Namibia School of Medicine, Ghana
Cite this as
Ababio GK, Adu-Bonsaffoh K, Abindau E, Quaye IKE. Lesser Weeks of Gestation Correlated with Levels of Cortisol in Ghanaian Women with Preeclampsia, Case-control Study. Arch Biochem. 2024;7(1):001-005. Available from: 10.17352/ab.000008Copyright License
© 2024 Ababio GK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.Following the different kinds of treatment of Preeclampsia (PE) that have challenged clinical regimens, it seems expedient to find ways of preventing PE. Cortisol might be the best factor and marker to provide the baseline information, hence the focus.
The aim was to determine cortisol levels in preeclampsia.
STROBE consensus checklist was utilized for this case-control study. Clinical information on 125 subjects (50 controls, 30 PE, 50 pregnant normotensive; with 5 lost by attrition) was obtained after ethical consideration (MS-Et/M.3 – P.3.2/2013 – 2014) and informed consent. Full blood count, lipid profile, and cortisol levels were analyzed after venipuncture and blood processing from subjects. SPSS version 20 was used to analyze data.
The mean cortisol level in PE was 149.37ng/mL and this was statistically significant when compared with non-pregnant normotensive (controls) only (p = 0.004); and likewise, with both pregnant normotensive and controls (p = 0.008). During cortisol stratification, the odds ratio between PE and controls was 0.0927 (p = 0.0001) while the odds between pregnant normotensives and controls was 0.0374 (p < 0.0001). A significant finding was seen between cortisol levels and lesser gestational weeks.
The fewer the weeks of gestation, the higher the cortisol level of the preeclamptic patient.
Introduction
Preeclampsia (PE) is defined as a multisystem disorder characterized by hypertension and proteinuria [1-4] and a leading cause of maternal mortality/morbidity worldwide. According to WHO, the maternal mortality ratio in Ghana is 350 per 100,000 live births and this is unacceptably high [5]. Among the factors that threaten neonatal survival due to early preterm delivery and prematurity [6] expectant management and placenta delivery (the rescue medical intervention) are of no exception.
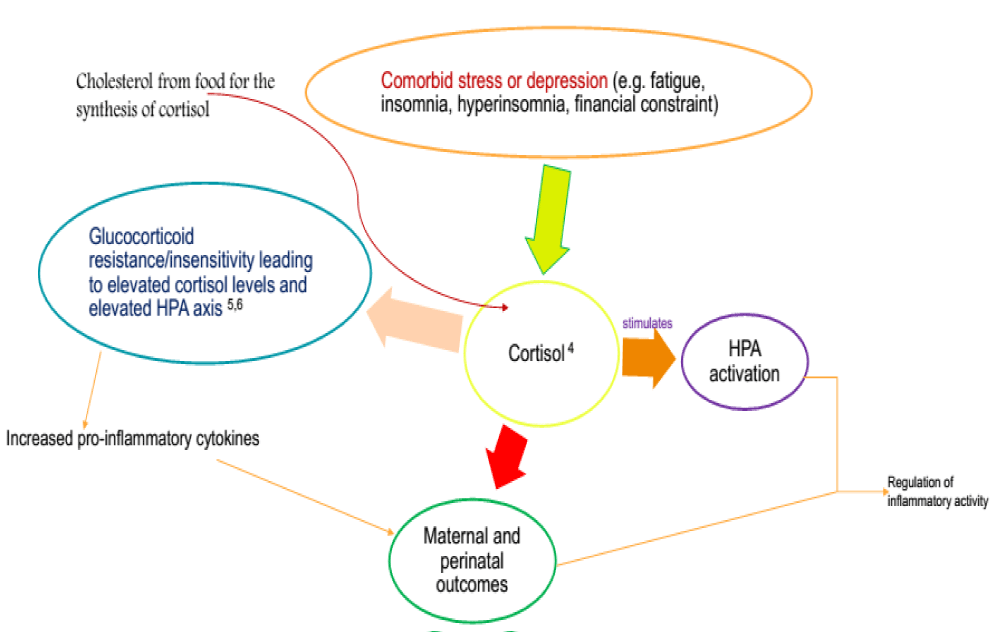
Other factors have also been cited to be responsible for the pathogenesis of PE [5-11]. The most prominent factors as far as this research is concerned, are stress and cortisol levels. Determination of cortisol levels may provide an additional platform (Figure 1) for data support. This is because cortisol is a stress mediator in the body and a possible instigator [7] of the major outcomes of preeclampsia but limited data exist worldwide.
Elevated cortisol levels during pregnancies have been suggested to be associated with unsuccessful pregnancies than successful ones [12]. Higher cortisol levels seem to be present in maternal circulation during pregnancy since it has been shown to control fetal pre-and postnatal development, affecting organ systems during cortisol programming [13]. If cortisol degradation is affected by an 11β - hydroxysteroid dehydrogenase type 2 (11β-HSD2) activity, then birth weight is lowered. Placental 11β-HSD2 is upregulated during the differentiation of cytotrophoblasts into syncytiotrophoblasts. However, if 11β-HSD2 activity is decreased first, a transfer of high cortisol levels into the fetal circulation would be achieved, thereby overwhelming the ability to degrade and adjust cortisol levels for current needs [14-18].
Our working model of cortisol’s influence on the hypothalamic (HPA) axis (Figure 1) of preeclampsia might provide evidence for policymakers. However, the specific role played by cortisol is not yet confirmed due to evolutionary influence e.g. heritable traits over successive generations and/or changes in maternal sensitivity to the environment that occur with advancing gestation. Additionally, there is evidence that maternal stress during pregnancy is related to decreased gestational length [18]. We explored the latter and asserted the notion that cortisol might be an additional marker to provide baseline information in the prevention and management of preeclampsia and its complications in Ghana.
Aim
To determine cortisol levels in the pathogenesis of preeclampsia
Method
Inclusion criteria
Patients diagnosed with Preeclampsia (PE) were recruited after informed consent. Non-pregnant women and pregnant normotensives who provided informed consent were also included.
Exclusion criteria
Any subject with a history of diabetes, chronic hypertension, urinary tract infection, renal disease, cardiovascular disease, molar pregnancy, multiple pregnancies, infectious diseases, and thyroid dysfunctions were excluded. Less than 18 years females were also excluded.
Clinical information was obtained after informed consent and ethical review (MS-Et/M.3 – P.3.2/2013 – 2014) for the case-control and prospective study situated at the Obstetrics and Gynecology unit, Korle-Bu Teaching Hospital, Accra. The sample consisted of 125 subjects (50 controls, 30 PE, 50 pregnant normotensive; with 5 lost by attrition).
Four milliliter (4 ml) of blood was obtained (in the morning) from an antecubital vein by means of a plastic syringe and dispensed into EDTA (for complete blood count) and gel tubes taking careful precautions. Sysmex auto analyzer was used to quantify complete blood count whilst RANDOX auto analyzer was used for the determination of lipid profile.
Cortisol levels were determined using an ELISA kit from GenWay Biotech, Inc. (6777 Nancy Ridge Drive, San Diego, CA 92121).
Five mL spot urine was obtained to determine proteinuria and to categorize subjects into normotensives and PE.
PE in this study, was defined based on American College of Obstetricians and Gynecologist criteria. Accordingly, PE is defined as diastolic blood pressure of ≥ 90 mmHg and or systolic blood pressure of ≥140 mmHg with proteinuria ≥300 mg/dl occurring after 20 weeks of gestation.
Data was created on a spreadsheet, corrected for errors, three (3) different backups were created and SPSS version 20 was used for analysis.
Results
In preeclampsia, the average blood pressure ranged from early onset (141/90 mmHg; 20 weeks to ≤ 34 weeks) to late onset (157/94 mmHg, ≥ 34 weeks) respectively. The average blood pressure for pregnant normotensive was 111/74 mmHg.
Systolic blood pressure (SBP), Diastolic blood pressure (DBP), neutrophil count, white blood cell count, cholesterol, and LDL cholesterol were significant after both t-test and ANOVA analysis (Table 1). Early morning cortisol levels and other remaining parameters in Table 1 were only significant in the t - test analysis.
From Table 2, during cortisol stratification, the odds ratio between PE and controls was 0.0927, with a Confidence Interval (CI) being 0.0272 to 0.3152, a z score of 3.808, and a p = 0.0001. Even though the odds between PE and pregnant normotensive were not significant; the odds between pregnant normotensives and controls gave 0.0374, a CI being 0.0082 to 0.1717, z score of 4.228 and this was highly significant (p < 0.0001).
A significant finding was seen between cortisol levels and lesser gestational weeks (Table 3).
Inferences from cortisol levels stratification to control confounding clinical variables indicated how cortisol correlated with SBP, DBP, and glucose under normal conditions; however, results were not indicated here. This was partly due to low numbers obtained after stratification as some data was lost to attrition.
Discussion
In this study, a report on specific differences in cortisol levels in Ghanaian subjects was presented alongside our potential mechanism (Figure 1).
In agreement with the current finding, cortisol has been demonstrated to rise progressively during pregnancy with a peak during the second and third trimesters [19]. In this study, higher cortisol levels were found in preeclampsia patients with fewer weeks of gestation (around the second trimester) whereas the reverse was true in the third trimester for pregnant normotensive. With preeclampsia diagnosis at 20 weeks of gestation, it seems expedient to sample data from the second and third trimesters.
The study adds to the existing knowledge elsewhere that distress conditions during pregnancy, increase cortisol levels thereby altering the hypothalamic axis (HPA) [8-11,20], causing associated changes in cellular immunity and adverse pregnancy outcomes. Pregnancy outcomes due to abnormal cortisol levels could lead to fetal programming of organ systems [12] including metabolic and cardiovascular components. It suffices to say that cortisol correlated with SBP, DBP, and LDL cholesterol (Supplementary Table 1, though, there seemed to be data lost to attrition) as well as significant findings from the odds ratio (Table 2). It is also, possible that cortisol stimulated pro-inflammatory cytokines and elicited high blood pressure.
As pregnancy progressed, PE patients were less physiologically and psychologically reactive to stress, an indication of a dampening effect [20]. It is also not surprising that the study had a significant finding between cortisol levels and lesser gestational weeks (Table 3). In term PE patients, cortisol levels seem to be low before delivery and this was consistent with the literature [21,22]. This finding indicates that higher 11βHSD2 activity or lower 11βHSD1 activity in women with PE [20], perhaps, occurred before the clinical presentation. The inflammatory response and the associated stress in PE might increase cortisol secretion. However, this secretion does not constitutively increase in PE; and there is limited evidence that cortisol exerts a feedback inhibition or stimulates 11βHSD2 activity or whether other factors play a role - much work is needed in this regard.
Several investigators have suggested lower birth weight in the presence of decreased 11β-HSD2 enzyme activity [21-24]. This is very possible since a decrease in 11β-HSD2 could pave the way for higher cortisol levels to have a negative correlation. Even though patients were followed up until the delivery of babies, 11β-HSD2 enzyme activity was not explored due to logistics.
It is worth indicating that, pregnant normotensives and controls fairly filled out the stress-related questionnaire but only a handful of PE patients managed to complete that part of the questionnaire making the analysis a bit biased since PE was the main target. Thus, psychosocial parameter analysis was intentionally omitted in this article.
Conclusion
The fewer the weeks of gestation, the higher the cortisol level of preeclamptic patients and the need for mind-body intervention during preeclampsia. Further studies are needed in exploring the psychosocial relationship to cortisol/cortisone levels as well as addressing the effects of abnormal cortisol levels on hemodynamic states as an important modulator of 11β-HSD2 activity in preeclampsia, to inform decisions and the management of preeclampsia.
Recommendation
It is important to conduct longitudinal and intervention studies to improve maternal outcomes of PE.
We dearly acknowledge the following: CaRT, ORID - UG, Legon, Department of Obstetrics and Gynecology, PE patients, and the Study team.
Also, this work was presented briefly as a poster (PT0245) on Tuesday, September 27th, 2016, at the IASP 16th World Congress on Pain, Yokohama, Japan, September 26th - 30th, 2016.
Funding disclosure: Work from the authors’ laboratory was partly supported by grants from ORID - UG, University of Ghana Legon.
Data Availability: Data is available at Synapse with the main file ID being syn30132111, sub–file ID being syn30132164, and a running title as Cortisol and PE.
- Ababio GK, Adu-Bonsaffoh K, Botchway F, Abindau E, Quaye IKE. Hyperuricemia and adverse pregnancy outcomes in Ghanaian women: potential mechanism. Biochem Anal Biochem. 2016;5(2):275-279. Available from: http://dx.doi.org/10.4172/2161-1009.1000275
- Ababio GK, Tetteh D, Adu-Bonsaffoh K, Morvey D, Botchway F, Abindau E, et al. Comparative analysis of cardio metabolic profile before and after 34 weeks in Ghanaian preeclamptic patients. Mol Biophys Biochem. 2016;1(1):17-21. Available from: http://dx.doi.org/10.15761/MBB.1000104
- Ababio GK, Adu-Bonsaffoh K, Narh G, Morvey D, Botchway F, Abindau E, et al. Effects of lactate dehydrogenase (LDH) in preeclampsia. Clin Med Biochem. 2017;3:129. Available from: http://dx.doi.org/10.4172/2471-2663.1000129
- Ababio GK, Adu-Bonsaffoh K, Abindau E, Narh G, Tetteh D, Botchway F, et al. Effects of factor V Leiden polymorphism on the pathogenesis and outcomes of preeclampsia. BMC Med Genet. 2019;20(1):189. Available from: https://doi.org/10.1186/s12881-019-0924-6
- Srofenyoh E, Ivester T, Engmann C, Olufolabi A, Bookman L, Owen M. Advancing obstetric and neonatal care in a regional hospital in Ghana via continuous quality improvement. Int J Gynecol Obstet. 2012;116(1):17-21. Available from: https://doi.org/10.1016/j.ijgo.2011.08.010
- Gant NF, Cunningham GF. Management of preeclampsia. Semin Perinatol. 1994;18(2):94-102. https://pubmed.ncbi.nlm.nih.gov/8066479/
- Marcus SM. Depression during pregnancy: rates, risks and consequences. J Popul Ther Clin Pharmacol. 2009;16(1):e15-22. Available from: https://pubmed.ncbi.nlm.nih.gov/19164843/
- Berkenbosch F, van OJ, del RA, Tilders F, Besedovsky H. Corticotropin-releasing factor-producing neurons in the rat activated by interleukin-1. Science. 1987;238:524-526. Available from: https://doi.org/10.1126/science.2443979
- Avitsur R, Stark JL, Sheridan JF. Social stress induces glucocorticoid resistance in subordinate animals. Horm Behav. 2001;39(4):247-257. Available from: https://doi.org/10.1006/hbeh.2001.1653
- Ge M, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 2002;21(6):531-41. Available from: https://doi.org/10.1037//0278-6133.21.6.531
- Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11(9):625-632. Available from: https://doi.org/10.1038/nri3042
- Rajab K, Mohammad AM, Mustafa F. Increase cortisol in pregnancy. Int J Gynaecol Obstet. 2000;68:139-144. Available from: https://doi.org/10.1016/s0020-7292(99)00195-2
- Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal programming of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3(6):479-488. Available from: https://doi.org/10.1038/ncpendmet0515
- Schoof E, Girstl M, Frobenius W, Kirschbaum M, Repp R, Knerr I, et al. Course of placental 11beta-hydroxysteroid dehydrogenase type 2 and 15-hydroxyprostaglandin dehydrogenase mRNA expression during human gestation. Eur J Endocrinol. 2001;145(2):187-192. Available from: https://doi.org/10.1530/eje.0.1450187
- Hardy DB, Yang K. The expression of 11β-hydroxysteroid dehydrogenase type 2 is induced during trophoblast differentiation: effects of hypoxia. J Clin Endocrinol Metab. 2002;87(8):3696-3701. Available from: https://doi.org/10.1210/jcem.87.8.8720
- Odermatt A, Arnold P, Frey FJ. The intracellular localization of the mineralocorticoid receptor is regulated by 11β-hydroxysteroid dehydrogenase type 2. J Biol Chem. 2001;276(30):28484-28492. Available from: https://doi.org/10.1074/jbc.m100374200
- French NP, Hagan R, Evans SF, Godfrey M, Newnham JP. Repeated antenatal corticosteroids: size at birth and subsequent development. Am J Obstet Gynecol. 1999;180(1):114-121. Available from: https://doi.org/10.1016/s0002-9378(99)70160-2
- Reinisch J, Simon NG, Karow WG, Gandelman R. Prenatal exposure to prednisone in humans and animals retards intrauterine growth. Science. 1978;202:436-438. Available from: https://doi.org/10.1126/science.705336
- Glynn LM, Schetter CD, Hobel CJ, Sandman CA. Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health Psychol. 2008;27(1):43-51. Available from: https://doi.org/10.1037/0278-6133.27.1.43
- Jung C, Ho JT, Torpy DJ, Rogers A, Doogue M, Lewis JG, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96:1533-1540. Available from: https://doi.org/10.1210/jc.2010-2395
- Kajantie E, Dunkel L, Turpeinen U, Stenman UH, Wood PJ, Nuutila M, et al. Placental 11β-hydroxysteroid dehydrogenase-2 and fetal cortisol/cortisone shuttle in small preterm infants. J Clin Endocrinol Metab. 2003;88(1):493-500. Available from: https://doi.org/10.1210/jc.2002-021378
- Struwe E, Berzl GM, Schild RL, Beckmann MW, Dörr HG, Rascher W, et al. Simultaneously reduced gene expression of cortisol-activating and cortisol-inactivating enzymes in placentas of small-for-gestational-age neonates. Am J Obstet Gynecol. 2007;197(1):43-e1. Available from: https://doi.org/10.1016/j.ajog.2007.02.012
- Aufdenblatten M, Baumann M, Raio L, Dick B, Frey BM, Schneider H, et al. Prematurity is related to high placental cortisol in preeclampsia. Pediatr Res. 2009;65(2):198-202. Available from: https://doi.org/10.1203/pdr.0b013e31818d6c24
- Jayasuriya NA, Hughes AE, Sovio U, Cook E, Charnock-Jones DS, Smith GC. A lower maternal cortisol-to-cortisone ratio precedes clinical diagnosis of preterm and term preeclampsia by many weeks. J Clin Endocrinol Metab. 2019;104(6):2355-2366. Available from: https://doi.org/10.1210/jc.2018-02312

Article Alerts
Subscribe to our articles alerts and stay tuned.
 This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International License.

 Save to Mendeley
Save to Mendeley
